CIHR Internal Assessment - Report for the 2011 International Review
Part 1: CIHR, the Organization
Governance and management
As a federal agency, CIHR reports to Parliament through the Minister of Health and is a part of Health Canada's Health Portfolio. However, CIHR is governed by its independent Governing Council (GC) of 18 members (including the President) who are appointed by the Governor General of Canada on advice from the Cabinet of Canada (the federal cabinet). GC (Figure 1) is responsible for setting the overall strategic directions for CIHR and approving its budget. As part of its overall responsibility for evaluating CIHR's performance, GC commissioned this international review and will receive the IRP's report. GC also appoints the institute scientific directors (SDs) and members of the Institute Advisory Boards (IABs). Before the establishment of the Scientific Council in 2007, members of GC were primarily distinguished health researchers. Now, as vacancies arise, GC will evolve into a true corporate board through the appointment of a broader range of Canadians such as health system managers, health institution managers, senior administrators from academia, industry, governance and ethics experts and health-policy makers. The Deputy Minister of Health (a civil servant) is an ex-officio, non-voting member. Six subcommittees report to GC: the Executive Committee, the Standing Committee on Finance and Planning, the Governance and Nominating Committee, the Audit Committee, the Standing Committee on Ethics and the Stem Cell Oversight Committee. Where specialist knowledge is advantageous, additional subcommittee members may be recruited from outside GC. The President is both the CEO of CIHR and the Chair of GC, but the vice-chair of GC assumes the Chair at meetings to allow the President to fully participate in discussions. GC usually meets three times a year and has an annual strategic retreat.
Figure 1: CIHR organizational model
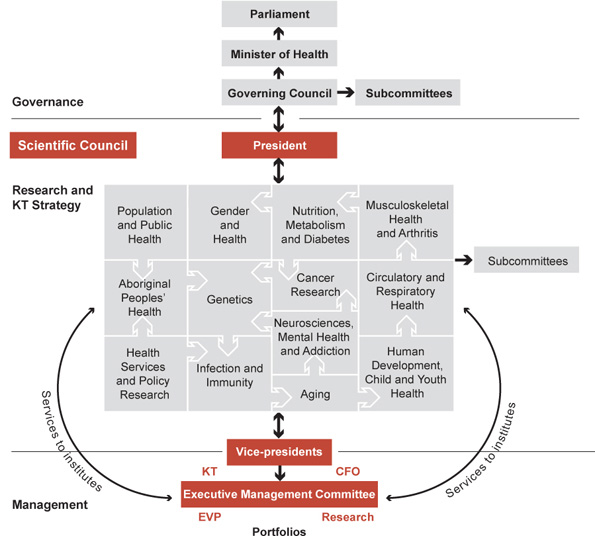
The Scientific Council (Figure 1) is the highest-level decision-making forum for science strategy and funding decisions. It is chaired by the President and composed of the 13 institute scientific directors (SD), the vice-presidents, the Director of Ethics, and two non-voting members: the Chief of Research Operations and the Director, Marketing and Communications. Scientific Council meets monthly and provides scientific leadership and advice to GC on health research and KT priorities and strategies, in accordance with the overall strategic directions determined by GC.
Table 1: Executive Management Committee
| Position | Focus of Responsibility | Portfolio Functions |
|---|---|---|
|
Executive vice-president (EVP) |
|
|
|
VP research portfolio and chief scientific officer (Research) |
|
|
|
VP resource planning and management portfolio and chief financial officer (CFO) |
|
|
|
VP knowledge translation and public outreach portfolio (KT) |
|
|
A key role for Scientific Council is the selection and development of large multi-institute strategic initiatives. To ensure consistency and increase the evidence base for decision making, there has been a recent shift from ad hoc decision making to a regular annual cycle. In March, Scientific Council selects from ideas put forward by its members. The champion(s) of each potential initiative write a concept paper outlining its rationale, scope and alignment with CIHR priorities. If this passes scrutiny, a detailed business plan is developed over the following months. If the plan is subsequently approved, funding is allocated from future-year budgets, relevant funding competitions are planned and a funding opportunity is posted on the CIHR website. The entire process from conception to funding can take more than two years but, to deal with unanticipated events, the President can sanction a fast-track process.
Scientific Council has three subcommittees: Performance Measurement, Planning and Priorities and Management (agenda setting and governance). The day-to-day business of the CIHR corporate office is managed by the President and his executive team (Table 1) who form the Executive Management Committee (Figure 1).
The CIHR Institutes
The institutes lie at the heart of CIHR and are its distinctive and fundamental organizational feature. The slate of 13 institutes adopted in 2000 (Figure 1) was intended to cover the entire universe of health research, with no significant areas, disciplines or issues excluded. Indeed, during the first 10 years of CIHR, every research issue that has arisen has been championed by one or more institutes. The slate is a combination of body systems, disciplines, targeted populations, diseases and themes. Although each institute should address research in all four themes as envisioned at the inception of CIHR, two institutes (the Institute of Health Services and Policy Research and the Institute of Population and Public Health) have mandates chiefly aligned with themes 3 and 4, respectively. As a result, these two institutes have had the added responsibility of serving as theme champions to help the other institutes meet their theme 3 and 4 mandates.
The SDs who head each institute are recognized leaders of the cognate research community, seconded from regular responsibilities while remaining at their home institutions, and devoting nominally 50% of their time to research, though many dedicate a greater proportion to institute responsibilities. The SDs are each assisted by a small staff, some located in Ottawa, some at their home institutions, to monitor the capacity and performance of the research areas within their institute mandate, develop and manage specific targeted research initiatives, form and maintain partnerships for research funding and KT activities, and evaluate outcomes and impacts of the research within the mandate. SDs are aided by the corporate office of CIHR in Ottawa, which exists to support the institutes, for example, with additional expertise on KT tactics and communications, and by providing funding competition, peer review and grants administration services. The corporate office also provides consistency and coordination in policy and ethics matters, international relations, the rules of engagement for partnerships and communications.
Each institute has an ~16-member (to be decreased to 14 in 2011) volunteer IAB primarily composed of researchers, but including some members from the public, private and non-profit sectors, including health practitioners and health care system decision and policy makers. The IABs help the SD draft the institutes' strategic plans (consistent with the overarching CIHR plan), set and evaluate the institutes' research priorities and allocate their research budgets accordingly. Through the IABs and the subcommittees of the Governing and Scientific Councils, CIHR benefits from a constant stream of timely advice from leading researchers and other stakeholders in health research. With the Scientific Council as the decision-making body with respect to strategic initiatives, IABs have been shifting their attention from details of institute budget allocation and programming to providing more strategic direction and advice about partnerships and collaborations and measuring the impact of the institute.
The institutes have played a pivotal role in transforming the health research landscape that CIHR inherited from the Medical Research Council of Canada (MRC). They have been instrumental in identifying priority health problems or neglected areas where there was a need to build research capacity – and to encourage research in those areas through strategic initiatives supported by dedicated funding. Through the development of judicious partnerships and a close understanding of the community and stakeholders, the institutes have leveraged funds and mobilized talent to support research priorities in their fields. Through the mix of approaches required to address these priorities, they have contributed to increased research funding and capacity, particularly in themes 3 and 4.
Although each institute research budget is relatively small (~$8.5 million per year), the strategic use of funds – individually or in collaboration with other institutes and corporate portfolios – has enabled institutes to invest in neglected or emerging areas of health research, generating new knowledge, building research capacity and developing competence, so that researchers working in these areas can go on to secure continuing support from CIHR's open funding competitions.
Furthermore, through their membership on Scientific Council, the institutes enable CIHR to reach consensus when it chooses large, multi-institute health research initiatives. While the institutes do not individually control the detailed allocation of most of CIHR's budget, collectively they exert the determining influence on the strategic agenda of CIHR and its resultant research spending.
The institutes also add value to CIHR by providing specialist scientific acumen as well as the viewpoints of their research communities and relevant stakeholders in health research. They determine the priorities for targeted research and areas where research capacity needs to be built through strategic initiatives. Existing outside the federal bureaucracy, institutes can act nimbly in response to emerging health threats, as during the 2003 severe acute respiratory syndrome (SARS) and 2010 porcine influenza (H1N1) outbreaks and the 2010 medical isotope shortage. By sponsoring workshops and symposia, institutes create a sense of community among the geographically scattered researchers already interested in the health problems that fall within their mandates. They also attract others to become involved, including funding partners and knowledge users. Institutes create a meeting ground where collaborations can develop with individuals and organizations who share common interests.
CIHR's position within the federal research and innovation system
CIHR is one of three federal research granting councils (Figure 2), known as the Tri-Council. The other two councils are responsible for the funding of academic research in the natural sciences and engineering (the Natural Sciences and Engineering Research Council, or NSERC), and in the humanities and social sciences (the Social Sciences and Humanities Research Council or SSHRC). In addition to bilateral partnerships in specific interface areas, such as the joint CIHR–NSERC program that supports collaborative research projects between natural sciences and engineering and the health sciences, the Tri-Council cooperates in funding programs that span the entire range of research disciplines, such as the Networks of Centres of Excellence (NCE), the Centres of Excellence for Commercialization of Research (CECR), the Canada Excellence Research Chairs (CERC), the Canada Research Chairs, (CRC) and the Banting and Vanier studentships. They also cooperate in matters of common policy interest such as ethics and research integrity.
CIHR also collaborates closely with four other federally-funded independent agencies that are important supporters of health research (Figure 2):
-
Genome Canada (established in 2000) supports large-scale genomics and proteomics research projects and regional research platforms.
-
The Canadian Health Services Research Foundation (CHSRF) (established in 1997) pioneered the science and practice of KT and knowledge exchange in health research in Canada.
-
The Canada Foundation for Innovation (CFI) (established in 1997) provides support for equipment and infrastructure. (For both foundations, approximately half or more of their investments flow to health research.)
-
The International Development Research Centre (established in 1970) helps developing countries use science and technology to find solutions to their social, economic and environmental problems, and is a key partner with CIHR on global health activities.
-
The Indirect Costs Program (established in 2003) helps alleviate the financial pressures on research being conducted in Canadian postsecondary institutions by providing support for overhead costs, salaries for staff or students who provide research administration support, training costs for workplace health and safety, and other administrative costs.
Whereas CIHR reports to Parliament through the Minister of Health (Figure 2), the other granting councils report through the Minister of Industry. Federal science and technology policy is the responsibility of the Minister of Industry.
Figure 2: The federal research and innovation system
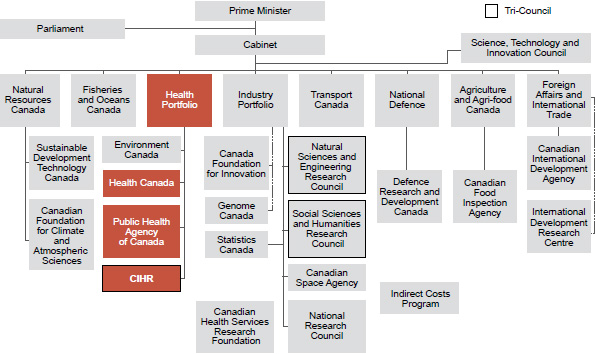
Adapted from Community Research and Development Information Service web site
Many federal science-based departments and agencies perform intramural research; CIHR has engaged in research or other partnerships with almost all of them.5 The largest is the National Research Council, comprising more than 20 bricks-and-mortar institutes across Canada, with a focus on technology development and commercialization.
The Science, Technology and Innovation Council, established in 2007, provides high-level advice to the Cabinet of Canada through the Minister of Industry. Composed of distinguished researchers, leaders of high-tech industry, university leaders and senior civil servants, it determines the priority areas for Canada's science and technology strategy, and issues periodic reports on Canada's performance in research and innovation, the first in 2009.6
The Government of Canada's Health Portfolio
The Health Portfolio consists of three major and three minor agencies. The three large agencies are as follows:
- CIHR
- Health Canada. This agency protects Canadians against risks from the environment, ensures the safety of consumer and health products, and is responsible for the approval of new drugs. It is also responsible for delivery of health care to First Nations people on reserves and to Inuit communities in the North.
- The Public Health Agency of Canada. This agency created in 2004 following the SARS outbreak, focuses on health promotion and prevention of chronic disease, health and disease surveillance, and is responsible for infectious disease control and the response to public health emergencies. It works with provincial, territorial and municipal governments, which share the responsibility for protecting public health.
There are regular information and coordination meetings between the civil service heads of the three large agencies.
As a federal agency charged with supporting health research and ensuring that the results are applied, CIHR through its institutes has strong links with the 13 provinces and territories responsible for public health and health care delivery to Canadians. In Canada, the provision of health care services is primarily a matter of provincial or territorial jurisdiction, with the federal government contributing to provincial and territorial health spending through transfer payments while also providing health care to Aboriginal peoples, the military and prisoners. Provinces and territories receive federal transfer payments (currently at $38.5 billion per year) if they abide by the five principles of the Canada Health Act (universality, comprehensiveness, portability, accessibility and public administration), which is the legislation that governs Medicare, Canada's universal health insurance program for physician and hospital services. The provision and organization of services that fall outside of Medicare – including most pharmaceuticals, longterm care, dental care and more – is up to each individual province or territory. This reality has contributed to Canada's unique suite of more than 13 health care systems. Financing is usually from a mix of public and private sources. As with many other Organisation for Economic Co-operation and Development (OECD) member countries, total health expenditures in Canada have increased at a rate that exceeds growth in GDP.
Rapidly rising costs for health care are a concern for the provinces, which already allocate 40% to 50% of their budgets for this purpose. As a result, there has been increased collaboration between the federal, provincial and territorial governments in health care policy. For example, the Common Drug Review process now involves the Government of Canada and all provinces (except Quebec) and provides recommendations for or against the inclusion of new drugs in provincial formularies. Discussions are in progress among the provinces to establish a national purchasing agency for drugs and medical supplies. Despite these improvements in coordination, it remains a challenge for the 13 independent health care systems to work together to ensure that a research finding on, for example, improvements in stroke care delivery in rural Nova Scotia is disseminated to and adopted by health care managers in rural Saskatchewan. Many medical services are outside the scope of the Canada Health Act (e.g., home care, dental care), and there are differences among provinces in the public insurance coverage for such services.
Integration of CIHR within the Canadian health research landscape
CIHR's success depends on partnerships with other participants in Canadian health research. Foremost are the universities, hospitals and research institutes where health research is performed. These institutions contribute the salaries of the investigators who receive CIHR grants, and provide and service their work spaces. CIHR maintains close relations with the
Association of Canadian Academic Healthcare Organizations, the national association of research hospitals, academic regional health authorities and their research institutes.
Most provinces have health research funding agencies, the largest being in Quebec (Fonds de la recherche en santé du Québec, or FRSQ, with a budget of ~$100 million in 2008–2009),7 Alberta (Alberta Innovates – Health Solutions)8 and British Columbia (Michael Smith Foundation for Health Research). Ontario has no comprehensive health research funding agency, but through its Ministry of Research and Innovation supports a number of organizations and programs. In 2003, the provincial agencies formed the National Alliance of Provincial Health Research Organizations (NAPHRO) as a forum for discussion of common issues.
The 27 largest health research charities are members of the Health Charities Coalition of Canada and CIHR, through its institutes, has partnered with most members. Significant mutual advantages to such partnering include pooling resources for joint research priorities, reducing duplication, increasing opportunities for KT, showing CIHR and health researchers to be responsive to citizen health concerns, engaging those affected by health issues in developing the research agenda and assisting charities with their fundraising for research. CIHR, the members of NAPHRO and the Health Charities Coalition meet twice annually at the Leaders Forum of health research funding agencies.
CIHR's KT mandate includes commercialization. Strong and ethical relations with the private sector are essential, and CIHR has regular discussions with BIOTECanada, representing the biotechnology industry, and Canada's Research-Based Pharmaceutical Companies (Rx&D), the umbrella organization for Canada's research-based pharmaceutical industry. The relationship with Rx&D is formalized in a joint funding agreement that has endured for almost 20 years and is currently being renewed. Since 2000, CIHR and Rx&D member companies have together invested about $360 million in research conducted in universities and hospitals.
Public advocacy groups for health research in Canada are small and under-resourced. Canadians for Health Research9 is the oldest and focuses on public information about the achievements of Canadian health researchers. Research Canada,10 primarily financed by several large hospital research institutes, is more oriented towards political advocacy. Friends of CIHR11 has a membership of distinguished Canadian researchers, active and retired, and publicizes health research through the awarding of a number of prizes.
- Date modified: