CIHR Internal Assessment - Report for the 2011 International Review
Part 5: The Way Forward
The implementation of Roadmap, CIHR’s new five-year strategic plan
In 2009, CIHR’s Governing Council approved CIHR’s second strategic plan (2009–2014) The Health Research Roadmap: Creating Innovative Research for Better Health and Health Care. This strategic plan is the product of widespread consultations with members of the health research community, careful assessment of CIHR’s strengths and weaknesses, and ongoing deliberation about what CIHR would like to achieve by 2014. Roadmap sets out a vision to secure Canada’s place on the world stage of health research for years to come. This vision includes four strategic directions.
Figure 22: CIHR’s Roadmap strategic plan
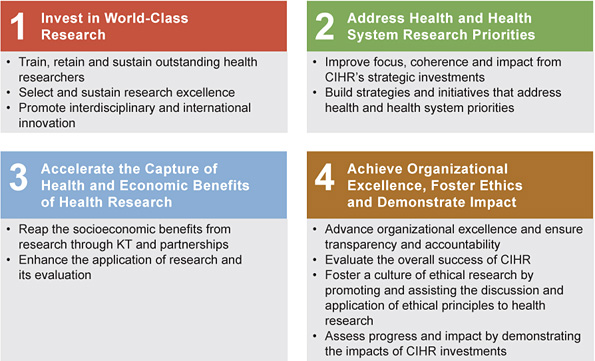
A rolling, three-year implementation plan has been developed for Roadmap. The implementation plan highlights activities CIHR will undertake over the next three years to implement the strategic plan. It also outlines some of the key results achieved in fiscal 2009–2010.
In January 2010, CIHR established the Roadmap Implementation Office. Its purpose is to support CIHR leadership in implementing the CIHR strategic plan, including the three reforms outlined below. The office is intended as a centre of expertise for implementation planning and change management. It provides Scientific Council and the Executive Management Committee with an integration and coordination point for all Roadmap implementation activities. It also provides implementation leads with proven methods to manage risk and change.
Reforms
To achieve its strategic directions, CIHR has identified three major reforms. These reforms will significantly affect the way CIHR achieves its mandate. They include reforms to CIHR’s open suite of programs, the peer review system and the strategic investment process. The interrelationship between the reforms is depicted below.
Figure 23: Inter-relationship of proposed reforms to achieve CIHR’s strategic direction

Reform of the peer review system
CIHR’s success at supporting excellence in health research depends on the quality of its peer review system. The first International Review Panel (IRP) noted the fatigue in an overworked system and, during the President’s recent cross-country tour, many researchers voiced concerns about the uneven quality of the review process. There is also a chronic problem recruiting committee members, in part due to lack of recognition for this vital service. CIHR is embarking on peer review reform that ensures all applications are evaluated with the same degree of rigour and fairness irrespective of research area or methodology, that adapts as research evolves and that makes optimal use of our most precious asset: the peers.
This reform is founded on the establishment of a College of Reviewers. The college will comprise accomplished Canadian and international researchers with expertise across the full spectrum of CIHR’s mandate, including knowledge users who can help judge the impact of research proposals. An invitation to join the college will be a mark of prestige, and acknowledged as such by CIHR and research institutions. College members will be supported by training, performance measurement and recognition programs. The college will enable a peer review system that is nimble and responsive to the varying nature of applications submitted to CIHR and sufficiently flexible to respond to the ever-changing nature of research. Furthermore, the college will reflect the recognition that a healthy, well- functioning, high-quality peer review system results from a collective effort where applicants, reviewers, funders, institutions and partners all contribute and benefit.
Reform of the open suite of programs
CIHR has a suite of open funding programs, open to all areas of health research and knowledge translation. Over the years several challenges have arisen, including an increasing number of applications and declining success rates in competitions for these grants, leading to wasted resources in applying for and peer reviewing them. In this context, four other challenges have arisen:
- Supporting truly innovative but risky proposals that are not backed by large amounts of preliminary data
- Giving talented and well-trained young investigators the chance to break into CIHR funding
- Supporting established, productive and creative investigators at a level that sustains their groundbreaking research programs
- Capturing excellence across all four themes of health research
The objective of reforming the open suite of programs is to design a core, stable suite of programs that can capture existing strength, capture ideas generated by an unrestricted applicant pool (individuals or teams), invite ideas across the full spectrum of health research to compete for funding, and rely on market forces to generate new ideas and new projects.
Reform of the process for strategic investments
In addition to the suite of open funding programs, CIHR also targets investments to address gaps in specific research areas or research communities and to leverage areas of strength in Canada. Over the years, several challenges have arisen, including the support of a large number of initiatives, which have limited the investment dollars available to each initiative. This reform responds to feedback from CIHR’s community for fewer, more targeted initiatives, the objective also being to attain greater focus in the use of strategic investments. CIHR’s targeted initiatives also need to work seamlessly with the new, integrated open suite of programs described above to ensure excellence and relieve the pressure on peer review. As part of the reform, a new annual process has been established recently to identify and plan targeted initiatives. The process is intended to concentrate limited resources on fewer better-funded initiatives, simplify the interface with CIHR for its partners and the research community and encourage multiple institutes and other branches across CIHR to collaborate on delivering strategic priorities.
This annual process starts with a scan of priority areas that outlines the level of investment in open and strategic programs and assesses the strengths, gaps and opportunities. This scan is carried out with input from all the institutes and their Institute Advisory Boards (IAB). Using the results of this scan, Scientific Council holds a priority-setting session. Once the target areas for investment are identified, a select number of concept papers that briefly outline key strategic initiatives are developed for review and approval by Scientific Council. The concept papers that are approved are then developed into business cases for funding decisions.
Enabling strategies
In addition to specific strategic initiatives, there are a number of strategies under development that can be thought of as enabling the overall research priorities of Roadmap. The diagram below shows the relationship between the research priorities and these strategies.
Figure 24: Current enabling strategies

Patient-oriented research strategy
This strategy was developed in response to a need for the Canadian health care system to embrace innovation and thus ensure sustainability and cost effectiveness.47 Canada has significant strengths in this area, including a high-quality health care system and research environment, a unique competence in systematic reviews, a record of research breakthroughs and high-impact clinical studies and population-based administrative databases as a basis for research.
The elements of the strategy are:
-
Improve Research Environment and Infrastructure. Support for People and Patient-Oriented. Research and Trials (SUPPORT) units will offer core research services to a region’s health system by supporting patient-oriented researchers and programs; educating and supporting health care professionals interested in evaluating the quality, accessibility and cost effectiveness of care, and developing new research programs; and implementing optimum standards for research involving human participants. In addition, research networks will be assembled to link SUPPORT units nationally across thematic areas such as mental health, primary health care and chronic disease management.
-
Set up mechanisms to train health professionals and non-clinicians in the core methods of clinical research and provide training for clinical epidemiologists, biostatisticians, methodologists and health economists, as well as research coordinators and project managers.
-
Strengthen organizational, regulatory and financial support for multi-site studies, and eliminate systemic barriers to patient-oriented research, such as the significant delays created by complex multicentre ethics review processes.
-
Support best practices in health care through collaboration between guideline developers and health care professionals. This will promote the development of high-quality, evidence-informed practice guidelines, and encourage policy makers, institutions, health care professionals and consumers to adopt them.
A critical issue in this strategy is the recruitment and retention of clinician-investigators in the face of economic and lifestyle disincentives. While the number of PhDs receiving CIHR salary support grew by 245 between 2004–2005 and 2008–2009, the number of health professionals grew by only eight. Although meeting this challenge is a commitment of Roadmap, it cannot be met by CIHR alone and will require investment from the provinces. In fact, the entire strategy will succeed only if provincial governments are engaged as equal funding partners and are prepared to put into practice the emerging research findings. This strategy is particularly timely because the pending adoption of electronic health records across the provincial health care systems is an opportunity to integrate them with databases of incomparable utility for health services and population health research.
Global Health Research Strategy
In partnership with the Canadian International Development Agency, Health Canada, the International Development Research Centre (IDRC) and the Public Health Agency of Canada, CIHR, led by the Institute of Population and Public Health (IPPH), has participated in the Global Health Research Initiative since 2001, spending more than $52 million in building collaborations between Canadian and low- and middle-income country researchers. A partnership with Grand Challenges Canada48 and IDRC has seen CIHR join the Grand. Challenges Board and provide peer review for the allocation of $225 million in federal funding over five years. This allocation is directed to five grand challenges, the first of which will create a new class of easy-to-use, low cost, point-of-care diagnostics. CIHR is one of six national research agencies49 that in June 2009 established the Global Alliance for Chronic Disease50 to fight chronic, non-communicable diseases by collectively developing a research base, as well as to develop and share best practices. Lowering hypertension, reducing tobacco use and indoor air pollution were chosen as initial priorities. In 2005, CIHR partnered with the Gates Foundation to support three Canadian teams that were successful in the Grand Challenges in Global Health Initiative competition, and a recently signed memorandum of understanding between the two agencies defines a framework for collaboration from 2010 to 2015.
The CIHR global health research strategy of January 2010 focuses on integrating global health into the activities of all institutes and programs, with a suggested focus on primary health care and strengthening health care systems. CIHR will seek national and international partnerships that enable it to have greater impact in pursuing global health goals that align with Roadmap priorities.
International Collaborative Research Strategy
Health research is increasingly performed by multidisciplinary, multi-investigator teams, often crossing the traditional boundaries of the Tri-Council. Therefore, CIHR must increasingly partner with other national and international funding agencies that share a common vision or set of priorities. Scientific Council has requested a strategy to provide guidance to institutes so they choose wisely among limitless opportunities for international collaboration. The following are current examples of international initiatives.
CIHR’s International Collaborative Research Strategy for Alzheimer’s Disease
This initiative is focused on risk factor identification, early diagnosis, early intervention and prevention of dementia. It will support translational, patient-oriented and health systems research that will in turn support a sustainable health care system for individuals with dementia. CIHR is a partner in the U.S. National Institutes of Health-led Alzheimer’s Disease Neuroimaging Initiative and provides financial support to the five Canadian sites. A funding partnership between CIHR, the Fonds de la recherche en santé, and the Agence Nationale pour la Recherche in France has operated since 2009. The German Centre for Neurodegenerative Diseases and the UK Medical Research Council have recently signed a cooperation agreement with CIHR to establish and apply harmonized guidelines and technologies for research on neurodegenerative diseases. A joint memorandum of understanding between CIHR and the National Natural Science Foundation of China is under development to support collaborative studies on the involvement of cerebral microvasculature in the pathogenesis of Alzheimer’s disease.
The Structural Genomics Consortium
This initiative is a public-private partnership that determines and publishes in the public domain ~150 3-D structures of proteins of biomedical importance each year. It operates out of the Universities of Toronto and Oxford and the Karolinska Institute. In addition to CIHR, it is funded by 12 other Canadian, UK and Swedish agencies, GlaxoSmithKline GSK, Merck and Novartis.
The Cancer Stem Cell Consortium
This consortium is composed of CIHR, Genome Canada, the Canada Foundation for Innovation, the Ontario Institute for Cancer Research, the Stem Cell Network, the National Research Council and the BC Michael Smith Foundation for Health Research. It is joining with the California Institute for Regenerative Medicine to fund multidisciplinary teams focused on cancer stem cell-based therapy.
Northern Research Strategy
This aligns with the Roadmap priority of decreasing health inequities in Aboriginal Peoples and other vulnerable populations. The health of northern peoples, particularly Indigenous inhabitants, is compromised by geography, lack of infrastructure and human resources, environmental and climate change issues, and cultural and social disconnection. As a result, the people of the Canadian North, particularly the Indigenous citizens, have the most compromised health in Canada. The strategy, led by the Institute of Aboriginal Peoples’ Health, will address knowledge gaps in three areas: access to health care in remote communities; climate change, food security and health; and the unique health challenges faced by First Nations, Métis and Inuit populations. CIHR hopes to involve Health Canada and the Department of Indian and Northern Affairs as partners, along with the three territorial governments and the provinces of Quebec and Newfoundland and Labrador. Collaborations with other circumpolar nations will build on the momentum established by the 2007–2009 International Polar Year and the 2009 International Congress on Circumpolar Health, held in Canada.
Future challenges
Supporting big science and research infrastructure
Increasingly, problems in the life and health sciences are being addressed through large, multinational research consortia such as the Human Genome Project, the SNP Consortium, and the International HapMap Project. These approaches challenge the traditional small-team, hypothesis-driven, experimental approach to biomedical science and may be resented by those who fear they will divert funds that could be invested in conventional operating grants. On the other hand, many of these consortia have been successful; membership in them keeps Canadian researchers at the forefront of the field and provides early access to improved technologies. For CIHR, which has an obligation to support a broad base of investigator-initiated research across Canada, a decision to invest significant funding in such "big science" consortia at a time of low budget growth is especially difficult, and Scientific Council has a regular process for reaching timely decisions on such opportunities.
CIHR is funding two large population cohort studies:
- The Canadian Healthy Infant Longitudinal Development (CHILD) Study51 is a study of 5,000 children born across Canada who will be followed from pregnancy until five years of age. The study will examine the influences of indoor air quality and its effect on the risk of asthma and allergies. The study is co-supported by the Allergy, Genes and Environment NCE.
- The Canadian Longitudinal Study on Aging (CLSA),52 initiated by the Institute of Aging, and now a major CIHR initiative, will follow 50,000 Canadians aged 45 to 85 for at least 20 years to understand how changing biological, medical, psychological, social and economic factors impact health and disability as people age. The CLSA is linked to other cohort studies around the world; it is essential that studies supported by CIHR provide unique information and contribute to international collaborative efforts. The study’s first five-year implementation phase is underway, supported by $50 million from CIHR, the Canada Foundation for Innovation and several provinces. An international oversight committee is monitoring progress and will advise CIHR on renewal funding.
CFI has transformed Canadian research institutions, enabling them to acquire state-of-the-art equipment and facilities. However, the foundation provides funds only for operation and maintenance of the equipment equivalent to 12% of the capital cost. Operating and maintaining the growing inventory of CFI-funded equipment to the end of its life-cycle is a growing challenge for research institutions, which look to CIHR for assistance.
Promoting and supporting data sharing and access
CIHR’s Policy on Access to Research Outputs, which came into effect January 1, 2008, requires that all research papers generated from CIHR-funded projects be freely accessible through the publisher’s website or an online repository within six months of publication. To complement the policy, CIHR, the National Research Council and the U.S. National Library of Medicine created PubMed Central Canada,53 where CIHR-funded researchers can deposit their peer reviewed research publications.
CIHR has established, in collaboration with Health Canada, the Drug Safety and Effectiveness Network,54 which will provide information on the safety and effectiveness of pharmaceuticals when used by diverse patient populations outside the controlled experimental environment of clinical trials. This endeavour is supported by $36 million over five years from the Government of Canada.
Access to provincially based administrative health data for research purposes is difficult: there are varying federal and provincial laws and regulations concerning privacy and consent. The Drug Safety and Effectiveness Network is working to establish a Canada-wide collaborating centre to access relevant administrative data. Given the recognition of drug safety and effectiveness as an essential element of protecting the health of the public, it is anticipated that access to administrative data for this purpose will be viewed more favourably by provincial governments than if it were intended only for investigator-driven research.
CIHR is involved with Genome Canada in efforts to establish the National Data Harmonization Platform, which would allow the pooling or the comparison of data-sets from CIHR-funded and other Canadian cohort studies. CIHR is also supporting international efforts to promote data sharing.55
Developing and evaluating multidisciplinary research teams
CIHR encourages multidisciplinary team approaches to research on complex health issues, but the formation, maintenance and evaluation of such teams has proven challenging. Formative evaluations have emphasized the creativity that emerges from such teams, the rich experiences for trainees and the advantages they have for KT. However, they have also underlined the difficulties experienced when researchers from different disciplines work together. It takes about two years before new teams run smoothly, meaning the standard, normal, non-renewable five years of funding for new teams may be too short to yield maximum impact from the team’s efforts. Other issues include ensuring that multidisciplinary applications for funding are expertly and comprehensively reviewed and establishing individual credit for team accomplishments.
The institutes are facilitating the work of multidisciplinary teams by providing more advice and training to the leaders and participants, and many now bring funded teams together for workshops at the start and at intervals during the duration of funding. This enables teams to discuss their successes and challenges and learn from each other.
Improving commercialization and relationships with the private sector
Given the adverse economic conditions for research investment by the life sciences industry, it will be especially challenging for CIHR to achieve the Roadmap goal of increased commercialization of health research. With the assistance of its Commercialization Advisory Committee, CIHR will put more effort into establishing liaisons with companies and exploring the advantages of participation in CIHR’s matching research programs. These may need to be redesigned to better serve the current needs of industry and the venture capital community. University technology transfer offices, the gatekeepers to intellectual property developed by CIHR grant holders, will also have to be engaged in these discussions and supportive of changes.
Finding new approaches to drug development
Inefficiencies, duplication and a high failure rate characterize the current system of drug discovery. It has been argued that "clinical proof-of-concept studies for selected targets should no longer be considered as a step on the path to commercialization, but rather as a precompetitive scientific experiment whose output can therefore be made available to all, without restriction on use,"56 leading to a proposal for an academic–industry consortium that would develop clinical probes of validated efficacy in humans for a wide array of potential targets. A public-private partnership model for the funding and development of innovative, precompetitive research activities already exists in the Quebec Consortium for Drug Discovery.57 Extending this model to a nationally-based consortium involving other organizations, such as the Centre for Drug Research and Development in Vancouver and MaRS Innovation in Toronto, would harness collective Canadian expertise and make Canada more attractive to international pharmaceutical investment.
Increased pressure for CIHR funding
A welcome federal investment in personnel, training and infrastructure increases the demand for operating funds from CIHR. A 2007 study forecast increased funding pressure on CIHR equivalent to $400 million by 2010 due to these additional investments in health research.58 The proportion of federal funding that supports the operating costs of research has fallen from almost 70% in 1997–1998 to less than 50% in 2007–2008. Two changes will further increase the pressure on applications to CIHR. First, the Canadian Health Services Research Foundation is moving away from research granting and may expend its endowment over the next few years. Second, the Social Sciences and Humanities Research Council (SSHRC) announced in May 2010 that it would exclude research that is primarily intended to improve health, health services and products or the Canadian health care system. In 2008–2009, SSHRC was supporting 330 health-relevant grants and awards. Many of these researchers will now look to CIHR for support.
- Date modified: