Review of the Institute of Genetics (IG)
Report of the IG Review Panel
February 2018
At the Canadian Institutes of Health Research (CIHR), we know that research has the power to change lives. As Canada's health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
Canadian Institutes of Health Research
160 Elgin Street, 9th Floor
Address Locator 4809A
Ottawa, Ontario K1A 0W9
This publication was produced by the Canadian Institutes of Health Research. The views expressed herein do not necessarily reflect those of the Canadian Institutes of Health Research.
IG Review Panel:
Chair: Daniel Durocher, Senior Investigator, Lunenfeld-Tanenbaum Research Institute, Canada.
Panel Members:
- Bartha Knoppers, Professor and Director, Centre of Genomics and Policy, McGill University, Canada.
- Monica Justice, Professor of Molecular Genetics, Program Head and Senior Scientist, University of Toronto, Canada.
- Durhane Wong Rieger, President and CEO of the Canadian Organization for Rare Disorders, Canada.
- Han Brunner, Professor and Head of Clinical, Maastricht UMC+, Netherlands.
- David Valle, Director, Institute of Genetic Medicine, Johns Hopkins University, USA.
Thanks to all participants in this review and the IG CIHR Evaluation Team: Ian Raskin, Michael Goodyer, Doaa Saddek, Kim Gaudreau, Jonathan Gilbert, Jean‑Christian Maillet, Sheldon Polowin, and Carole Chow.
And special thanks to:
- Dr. Paul Lasko, Scientific Director, IG;
- Dr. Étienne Richer, Associate Director, IG; and
- Dr. Eric Marcotte, Associate Director, IG.
For more information and to obtain copies, please contact: Evaluation@cihr-irsc.gc.ca.
Table of Contents
- Executive Summary
- Overview of the Review
- Observations and Recommendations
- Review Key Findings
- References
- Appendices
I. Executive Summary
The review of the Institute of Genetics (IG) was undertaken by the Canadian Institutes of Health Research (CIHR) as part of the review of the mandate and performance of CIHR Institutes by CIHR’s Governing Council (GC) outlined in the CIHR Act. The review assessed the relevance and performance of IG to inform decisions regarding the role and functioning of the Institute. The review was conducted by the CIHR Evaluation Unit and overseen by a panel of experts in IG’s mandate areas who reviewed and interpreted the findings and made the final recommendations. The recommendations and observations of the Panel are summarized below in relation to the two broad issues addressed by the review.
Are changes needed within the current IG mandate in order to address emerging areas of research?
The Panel concludes that IG’s mandate is appropriate given the fundamental and central role of genetics in biology and medicine. The Panel recommends that the Institute continue with its current mandate.
The Panel recommends that the next SD direct more attention to computational biology and computational medicine as these areas will revolutionize medicine. It is advisable that these areas be stressed as priorities under the next Institute Strategic Plan. It is imperative that the new SD is able to shape IG’s priorities in the early part of his/her mandate.
Furthermore, given the breadth of the IG’s mandate and the limited resources currently available, more discretion should be granted to the Institute to select and focus on certain aspects of the current mandate. The Panel recommends that CIHR commit an additional $4 M per year to support new investments in areas of strategic priority given the breadth of IG's mandate and available Institute resources.
Observations and Recommendations for the Next Scientific Director
As the current SD of IG will complete his second and final term in June 2018, the Panel provides advice to GC and CIHR to inform the transition of the Institute and the work of the next SD. The current IG SD has demonstrated strong skills in engaging the research community and is well-regarded by both researchers and stakeholders.
The Panel feels strongly that the next SD should be an active and respected scientist. The Panel agrees that the next SD needs to have the ability to manoeuver and quickly respond to emerging areas within the Institute’s mandate and this would be facilitated by the establishment of the proposed innovation fund. The Panel recommends that the next SD, in consultation with the IAB, develop a strategy to assess the ongoing research priorities in order to ensure that emerging areas under the IG mandate can be prioritized. Additionally, the next SD will need to access a broad domain of expertise across IG’s mandate as well as in the areas of new and early-career investigators, and training of scientists and clinicians. The Panel recommends that the next SD develops an effective communication strategy about the mandate and funding opportunities sponsored by IG with a particular emphasis on early career investigators. The Panel recommends that the IG explores ways to increase involvement of early career investigators in their funding opportunities.
II. Overview of the Review
A. Institute of Genetics: Background
As one of the 13 CIHR Institutes, the IG has a vision to maximize the health opportunities offered by genetic, basic biochemistry and cell biology research to the benefit of Canadians and the global community by advocating for and supporting the Canadian genetic community both nationally and internationallyFootnote 1.
IG’s mandate is to support research on the human and model genomes and on all aspects of genetics, basic biochemistry and cell biology related to health and disease, including the translation of knowledge into health policy and practice and the societal implications of genetic discoveries. Within its mandate, IG supports capacity building in addition to emphasizing knowledge translation and exchange into health policy and practice, and developing a comprehensive understanding of the societal implications of genetic discoveries.
B. Review Objectives
The review of the IG was conducted by CIHR as part of the ongoing assessment of the mandate and performance of the 13 CIHR Institutes. The review assessed the relevance of the mandate of IG and the performance of the Institute in order to inform decisions regarding the role and functioning of the Institute and the transition of the Institute to the next SD. The aim of the review is to provide the GC with valid and reliable findings to inform decisions regarding:
- Whether changes are needed to the current IG mandate in order to address emerging areas of research; and,
- Observations and considerations for the Institute transition and next SD.
The review was overseen by the IG Review Panel (hereafter referred to as the Panel) comprised of experts in the IG mandate areas and conducted by the CIHR Evaluation Unit. The names and affiliations of the Panel members are listed in Appendix 1.
The review covered the period 2000-2016, with a focus on the period under the leadership of the current SD, Dr. Paul Lasko, from 2009 and 2016Footnote 2. The review was informed by a review of documents and data, interviews with the IG stakeholders, and a bibliometric study of the publications of Canadian researchers within the field of genetics. The methods and data sources used are outlined in Appendix 2 and key figures are presented in Appendix 3. While each line of evidence has limitations, there is convergence among them so as to produce key findings for the review.
III. Observations and Recommendations
A. Are changes needed within the current IG mandate to address emerging areas of research?
1. Context
As outlined in the CIHR Act, the objective of the CIHR is:
“to excel, according to internationally accepted standards of scientific excellence, in the creation of new knowledge and its translation into improved health for Canadians, more effective health services and products and a strengthened Canadian health care system…”Footnote 3
Among the many activities to achieve its objective, CIHR is responsible for “encouraging innovation, facilitating the commercialization of health research in Canada and promoting economic development through health research in Canada.” And, as divisions within CIHR, the Institutes are expected to contribute to the achievement of CIHR’s overall objective within their mandate through a number of activities, including: “work in collaboration with the provinces to advance health research and to promote the dissemination and application of new research knowledge to improve health and health services.”
The Panel agrees that the IG’s mandate is appropriate given the fundamental and central role of genetics in biology and medicine, and the clear progression from discovery to translation. However, IG is challenged by an expansive mandate relative to the size of its budget. The SD is required to balance his emphasis, timing and evaluation in consideration with the current scientific context and the funding pressure. In addition, partnership with the private sector and commercialization is a challenge for IG as well as for the other CIHR Institutes more broadly. One particular factor which clearly hampers commercialization efforts for IG is the absence of an orphan drug regulation in Canada, in contrast to the large majority of developed countries, which clearly stifles rare disease drug discovery and development, an area championed by IG.
IG’s current SD has demonstrated success, particularly in the area of rare diseases, where Canada is now recognized as an international leader working to catalyze and partner with the research and stakeholder community. IG co-leads the Personalized Medicine Initiative in conjunction with the Institute of Cancer Research (ICR) and Institute of Health System and Policy Research (IHSPR)Footnote 4, which includes major partnerships with the Cancer Stem Cell Consortium and Genome Canada. In 2012, CIHR, in partnership with Genome Canada, invested in the Large-Scale Applied Research Project (LSARP), which aimed to fund research projects focusing on the application of genomics in the area of precision health.Footnote 5 In addition, IG also co‑leads the Canadian Epigenetics, Environment and Health Consortium (CEERHC), with the Institute of Neurosciences, Mental Health and Addiction (INMHA) and ICR. The Initiative aims to position Canada for the rapid translation of epigenetic discoveries into practical health benefits.
Moreover, IG is committed to and supports capacity building through partnerships with other health organizations and professional associations, as well as by providing support to new investigators via holding an Annual New Principal Investigator (NPI) Meeting.
2. Scientific and Funding Landscape
The era of personalized and genomic medicine is upon usFootnote 6. Sequencing provides a diagnostic tool that allows for individualized treatment strategies. Indeed, the development and approval in the US of agents such as ivacaftor (Kalydeco) to treat cystic fibrosis and, more recently, luxturna (RPE65 gene therapy) to treat Leber congenital amaurosis mark important milestones in realizing the promise of genetics research for the treatment of diseases since these therapies target, in different ways, specific genetic defects. A similar drive towards personalized, genomics-based management of multiple other diseases is gaining pace, with oncology leading the wayFootnote 7.
A varied, bench-to-bedside, portfolio of research is necessary for the development of personalized therapies. The examples of ivacaftor and luxturna’s development are telling. From the initial identification of mutations in the CFTR and RPE65 genes, the development of these therapies necessitated a deep understanding of the molecular and cell biology of their gene products, research in chemistry, in virology, the development of models and then translation into products. The development cycle of personalized medicine dovetails remarkably well with the broad mandate of IG, which is to support research on the human and model genomes and on all aspects of genetics, basic biochemistry and cell biology related to health and disease, including the translation of knowledge into health policy and practice and the societal implications of genetic discoveries. Since personalized and genomic medicine has deep implications for the diagnosis, management and treatment of diseases that are the focus of most of the other CIHR institutes (diabetes, neuromuscular disorders, cancer, etc.). The IG should continue to take a leadership role, as it has taken with the Personalized Medicine/Health Initiative at CIHR.
The emergence of genomic data and other massive-scale “omics” approaches provides both opportunities and challenges for the biology and medicine of the future. How can one extract meaningful information from these multidimensional datasets, how can these datasets be readily accessible to a wide range of researchers, and what are the best practices to store this data? These issues are especially acute with respect to clinical and patient-derived data with their associated legal and ethical concerns. We encourage the IG to develop a broad-based strategy to ensure the continued development of the computational biology and medicine communities. It is advisable that these areas be stressed as priorities under the next Institute Strategic Plan.
The Institute is uniquely qualified to contribute to the development of health care policies and practices that will potentially improve the health of all Canadians. IG‑supported research is wide ranging, complex and innovative. Due to the growing importance of genetics in all branches of health research, IG facilitates and supports the genetic, biochemical and cell biology research initiatives of each of CIHR’s other 12 institutes. IG also organized the "DNA on Loan: Exploring Biobanking with Indigenous Values" forum to explore the issues surrounding long term storage of biological samples when research involves the Indigenous Peoples of Canada. The objectives were to provide national and international views on good practice and policy in this area and encourage dialogue that would provide background for enhancing current Canadian policy.
With respect to funding, the Canadian landscape of public funding of research under the IG mandate is dominated by CIHR investments in the investigator-initiated operating grant competitionFootnote 8 and by investments from Genome Canada, a non-profit corporation dedicated to harness the power of genomics for the benefit of CanadiansFootnote 9. With a comparably smaller budget, the IG needs to play a role of catalyst with strategically placed investments. The panel welcomed the level of cooperation between the IG leadership and Genome Canada in order to develop a strategic vision around genomics research.
3. Panel Observations
Achieving its mandate
CIHR’s budget has been effectively flat for approximately the last 10 years, and therefore it is declining substantially in real terms, greatly affecting the ability of researchers to sustain competitive research programs.
The Government of Canada’s 2017 Budget did not provide new, untargeted funding for the three federal research funding agencies: CIHR, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Social Sciences and Humanities Research Council of Canada (SSHRC).Footnote 10 The 2017 Budget established the position of Chief Science Advisor and related secretariat, funded the creation of approximately 25 Canada 150 Research Chairs and a number of investments to simplify and target support to Canadian innovators, including: Innovation Clusters and Networks, Impact Canada Fund, Strategic Innovation Fund, Venture Capital Catalyst Initiative, and Innovation Canada.
In terms of funding for CIHR, the 2017 Budget proposed funds for Health Canada, the Public Health Agency of Canada and CIHR to support measures associated with the Canadian Drugs and Substance Strategy ($100M over five years) and National Action Plan on Climate Change Adaptation ($47M over five years).
The final report of Canada’s Fundamental Science Review, released on April 10, 2017, stresses that significant reinvestment in the federal research ecosystem needed over a more predictable and better planned multi-year horizon as well as improved coordination and collaborations between the three federal granting agencies (CIHR, NSERC and SSHRC) and the Canada Foundation for Innovation (CFI).Footnote 11 The panel supports Canada’s Fundamental Science Review recommendations and their implementation would have a positive impact on the ability of IG to support its mandate. The 2017 Budget document indicates that the federal government will finalize its response to the advisory panel’s recommendations before making any new investments in the federal granting agencies, which could mean Budget 2018.
Until 2014‑15, each of the 13 CIHR Institutes received a strategic research budget of $8.6 M per year. As a result of the Institute Modernization, in 2015‑16, half of each Institutes’ strategic research budgets ($4.3 M per year) was invested in CIHR’s Roadmap Accelerator Fund (RAF) to support multi-Institute and multidisciplinary initiatives align with CIHR’s research priorities patterned along the lines of CIHR’s existing signature and strategic Initiatives. The remaining half of the budget remained under the control of Institutes to direct toward Institute-specific initiatives.Footnote 12 As of 2017-18, Institutes returned to a strategic research budget of $8.6 M; however, the investments of funds in multi-Institute and multidisciplinary initiatives are guided by the same “spirit” and principles as RAF. In the case of IG, the return to an $8.6 M strategic research budget will have limited impact on the available research for the next SD due to forward commitments to CIHR and Institute initiatives. For example, based on the current budget and commitments, the next IG SD will have a limited available research budget for much of their first four-year term: 2018-19 ($128.8 K), 2019-20 ($454 K); 2020-21 ($1.4 M); and 2021-22 ($2.1 M)Footnote 13. The lack of funds available for the next IG SD may hamper his/her ability to shape the IG’s priorities for many years of his/her mandate.
In addition to the IG budget, the total CIHR investment in the IG mandate research area (e.g. originating from investigator-led operating grants competitions) increased steadily between 2000‑01 and 2007‑08, from $118 M to $298 M, during a period when CIHR’s overall budget increased steadily, then decreased slightly until 2009‑10 before it increased over the following six years to reach $385 M in 2015‑16. For more information about CIHR investments in IG’s mandate by research areas,Footnote 14 see Figure A (Appendix 3).
In light of this, the Panel noted that offering each of the 13 CIHR Institutes a limited yet equal budget is not reflective of the reality of the scientific opportunity and importance of genetics to the domain of health research, nor does it reflect the broad mandate of the IG. As a consequence, the panel concludes that IG receives insufficient funding to effectively meet its mandate to support priority driven research and its translation. The Panel remarked that given the emergence of “big data” in every aspect of genetics, genetic medicine and health, this will be an important area of partnership for IG with Genome Canada, academic institutions, international organizations, patient groups and commercial partners. These areas also flag the need to focus on computational medicine and biology as priority areas under the Institute’s new strategic plan, given the limited resources.
Another upcoming challenge for IG is the SD transition. In particular, the change in staff allocation from Ottawa-based Institute staff (OBIS), who were personnel at CIHR’s central office dedicated to provide service to IG, to Integrated Institute Teams (IIT), who are providing support across all 13 Institutes undermines the ability of building the IG corporate memory, continuity and staff loyalty.
Furthermore, the Panel noted challenges with the assessment of IG’s performance in terms of knowledge translation and commercialization given it is a broader CIHR objective.
4. Recommendations
Recommendation 1: The Panel recommends that IG continue with the current mandate.
Recommendation 2a: The Panel recommends that the next SD direct more attention to computational biology and computational medicine as these areas will revolutionize medicine.
Recommendation 2b: The Panel recommends that CIHR commit an additional $4 M per year to support new investments in areas of strategic priority given the breadth of IG's mandate and available Institute resources.
5. Strategic Considerations
The Panel supports CIHR’s recent move to return to the 13 Institute-specific IAB model from the five IAB model aligned with the strategic directions and research priorities of CIHR’s five-year strategic plan, Health Research Roadmap II. The model is perceived to have diluted the domain expertise required by the SDs. The Panel advises that the new advisory board should include members with experience and knowledge of the international know-how. It should also be forward looking, diverse and covering all aspects of the IG mandate.
Moving forward, the Panel sees the need for a periodic assessment of the Institute’s strategic initiatives in order to ensure their success and their relevance and effectiveness and that they are meeting the needs of the research community.
B. Observations for the Next Scientific Director
1. Context
There is widespread appreciation for the current SD in the IG research and stakeholder community. IG’s current SD has been a tireless advocate and champion of rare disease and epigenetics research that led Canada playing a leadership role in these two areas. In addition, the current SD has been active building the networks (e.g. rare disease models) and developing collaborative initiatives. He also actively took on the responsibility of ensuring that the Institute is providing opportunities for the mentorship of new investigators. Furthermore, the current SD brought the Institute to the forefront in the patient networks. Throughout the SD’s term, the profile of the Institute has been brought to the forefront internationally.
The Panel expresses a need that the new IG leader be an active and well respected researcher with the following characteristics:
- Understanding of basic science;
- Understanding of the opportunities and challenges of translating science into clinical practice;
- Motivated toward translation and the application of research; and
- Strong communication and collaborative skills to be able to foster and maintain partnerships with other institutes, nationally and internationally.
The next SD should be able to elaborate an effective outreach and communication strategy that reaches the full spectrum of researchers working under the broad IG mandate. This strategy should pay particular attention to new investigators and early career scientists. Furthermore, the Panel agrees that the next SD needs to have the ability to both “step back” and set a clear strategic path for the Institute and be able manoeuver and quickly to respond to emerging areas and opportunities. For example, areas such as genome editing, cryoelectron microscopy or single-cell biology/genetics are revolutionizing research areas under the IG mandate. The Institute-specific IAB will be important for the next SD to help ensure he/she has the domain expertise required to develop and focus on the strategic priorities, and to obtain advice from and communicate with their Institutes’ research community.
Recommendation 3: The Panel recommends that the next SD, in consultation with the IAB, develop a strategy to assess the ongoing research priorities in order to ensure that emerging areas under the IG mandate can be prioritized.
Recommendation 4a: The Panel recommends that the next SD develops an effective communication strategy about the mandate and funding opportunities sponsored by IG with a particular emphasis on early career investigators.
Recommendation 4b: The Panel recommends that the IG explores ways to increase involvement of early career investigators in their funding opportunities. As one example, the IG could require the presence of early career investigators in team grants.
2. Panel Observations
The appointment of the IG’s next SD is crucial, since genetics and genomics is central to so many disciplines. After considering the activities, dedication and accomplishments of the IG’s current SD and the context within which the Institute is operating, the Panel concluded that the next SD should have the aforementioned characteristics.
IV. Review Key Findings
A. Review Objectives
The review of the IG was conducted by CIHR as part of the rolling review of all CIHR Institutes. The aims of the review are to:
- Provide CIHR management with valid, insightful, and useful findings regarding the ongoing institute relevance and performance; and
- Inform decisions regarding the transition of the Institute and the next SD.
Using a common framework of analysis, the review drew on multiple lines of evidence, including qualitative and quantitative data sources (Appendix 2). It uses administrative data on expenditures related to the IG mandates, bibliometric analysis on the ranking of Canada compared to the top active countries in the field of genetics research in IG’s priority areas, interviews with a number of IG stakeholders and Panel deliberations. While each line of evidence has limitations, there is convergence among them so as to produce key findings. Overall, we are reasonably confident that the results presented provide an accurate portrait of the relevance of IG’s mandate and the Institute’s performance.
The review was conducted by the CIHR Evaluation Unit and overseen by the Panel members who reviewed and interpreted the findings and made the final recommendations.
1. Relevance
Ongoing relevance of the IG mandate
The expanded areas of research that have emerged in the wider environment include an increased focus on Models & Mechanisms to Therapies, advances in Computational & Systems Biology, Strengthening the IG Research Community and significant Health Services as well as Policy & Ethics, Legal and Social issues.
The bibliometric analysis results showed how Canada ranks regarding IG’s priority research areas, namely computational biology, rare disease, precision medicine, Genetics-Ethical, Legal and Social Issues (GELS), epigenetics and genetics, when compared to the top 10 most productive countries in these research areas. The results provide a background concerning whether or not more investment is required in these areas moving forward to boost Canada’s position internationally. These results could also be of help to the next scientific director while developing and defining the Institute’s new strategic priorities.
Between 2011 and 2016, Canada’s ranking improved from the 7th to 6th concerning the number of publicationsFootnote 15 in the six priority areas accounting for an average of 5% of the world’s total annual publication. Results show that Canadian researchers are publishing in journals that are cited more often than the world average. This is more so for computational biology, genetics and epigenetics compared to the other areas. Between 2011 and 2016, the specialized index (SI)Footnote 16 shows that Canada is more specialized in the 6 priority areas compared to the world average. However, countries like UK, US and the Netherlands have consistently had higher SI than Canada over time.
There is a need for an institute that supports research on all aspects of genetics related to health and disease including the translation of knowledge into health policy and practice and the societal implications of genetic discoveries.
IG has been playing an integral role in developing, promoting and maintaining a research environment that addresses issues related to Genetics. Genetics research priorities are supported financially by CIHR more broadly and by IG specifically. From 2000‑01 to 2015‑16, the average annual percentage of CIHR investment in IG’s mandate was 32% of total annual CIHR investment. Figure A (Appendix 3) depicts CIHR’s investment in IG mandate by the Institute’s research priority areas, defined in its 2012-17 Strategic Plan. Figure A (Appendix 3) shows that from 2011‑12 to 2015‑16, the amount invested by CIHR in the “From Models & Mechanisms to Therapies” priority represents an annual average of 26% of total CIHR investment in IG mandate, compared to 7% in Computational & Systems Biology; 3% in Strengthening the IG Research Community; and 2% in Health Services, Policy & ELSI. From 2011‑12 to 2014‑15, CIHR’s annual investment in Models & Mechanisms to Therapies increased gradually and was always the highest compared to investment in the other research priorities.
When asked how effectively the Institute has met its mandate and current strategic priorities, the majority of the IG stakeholders interviewed highlighted that the Institute’s mandate covers important areas in the context of health of Canadians and the current SD has been striving diligently to support the research community under these areas of priority. Three of the interviewees were not sure what the mandate of the institute was, however.
Most of the stakeholders interviewed flagged that the IG mandate is very broad and while this important to make sure it covers important areas of genetics, the breadth of the mandate poses a challenge to the SD, given the limited resources at his/her discretion. As result the stakeholders interviewed see that there is a need for additional resources to address emerging areas of research in the field of genetics, most notably computational biology and medicine, precision health and precision medicine, and GELS, which the stakeholders deem as important and still need more attention from IG.
While the majority agrees that IG should invest more in funding basic science and research few highlighted that it would be better in the future to see that patient-engagement is made explicit in IG’s mandate.
2. Impact
Support to Innovative Research and Advancing Knowledge
The IG is committed to supporting innovative research and advancing knowledge. The SD is supportive of transformative research and is open to innovations, engages various stakeholders, is committed to client oriented research, and is an active researcher himself.
IG supported the integration of physical scientists and engineers into biomedical research. Between 2002 and 2011, IG invested nearly $20 M in biomedical research. The Institute co-led the Regenerative Medicine and Nanomedicine Initiative (RMNI), offered annual funding opportunities to foster the development of tools, techniques and devices, and organized several workshops. In addition it provided support to expand the GE3LS (Genomics and its ethical, environmental, economic, legal and social) research (basic & applied) community.
Moreover, IG has contributed to the 2012 Large-Scale Applied Research Project Competition (LSARP), which is one of the most significant public sector investments in Personalized Medicine. The outcomes of LSARP include: identifying recurrent driver mutations affecting DNA structure, now part of WHO test recommendations, developing an oncology panel which is now used in clinical trials and is certified by Clinical Laboratory Improvement Amendments (CLIA). Moreover, LSARP resulted in studying 845 different rare diseases, provided a diagnosis to over 1000 patients, identified 131 novel rare disease genes, developed three experimental therapies and contributed to international data sharing standards.
Contributions to Building Capacity of the Health Research Enterprise
IG engages in a number of capacity building activities, including Institute-facilitated capacity building events and other investments to maintain and strengthen research capacity in mandate areas. This is in part fostered by the SD, who is viewed as supportive of young investigators and invested in ensuring they have the proper mentorship and training to help them become independent investigators.
From 2009 to 2014, IG spent an annual average of 13% of its budget on capacity building, including investments in catalyst/pilot programs, training grants and awards, and development grants (Figure B, Appendix 3). As of 2015‑16, 33% of total CIHR funded direct traineesFootnote 17 (Figure C , Appendix 3) and 45% of indirect traineesFootnote 18 (Figure D, Appendix 3) were funded under the IG mandate.
Between 2012 and 2016, IG has invested around $1 M in postdoctoral fellowships under the Canadian Epigenetics, Environment and Health Research Consortium (CEEHRC).
IG dedicated substantial resources to capacity building, through the New Principal Investigator (NPI) Meetings, awards, and Strategic Training Initiative in Health Research (STIHR) grants, which sought to build capacity within Canada's health research community through training and developing researchers, fostering and supporting their careers.
IG’s stakeholders commended the effort made by the SD regarding raising the capacity of the research community; however, they flagged that the phasing out of the Clinical Investigator Program (CIP) was unfortunate as it left a gap in clinical fellowship. A few stakeholders mentioned that more investment needs to be made, moving forward, in raising the capacity of the new, early, and mid-career investigators. The stakeholders were concerned that the shortage of funding directed to those levels of researchers could ultimately lead to a brain drain. Although IG’s stakeholders praised the NPI, they highlighted that more work still need to be done to improve communications with the new investigators especially to increase their awareness of and access to funding opportunitites of IG and within IG’s mandate.
3. Convener and Catalyst
Contribution of Scientific Leadership to the Convener-Catalyst Role
There was a consensus amongst the stakeholder interviewed that IG’s SD was very active in building and maintaining partnerships and collaborations. He has engaged in a variety of activities to identify, connect and engage with multiple organizations, and has participated in events (i.e., workshops, meetings and conferences) where he spoke to IG’s strategic priorities and to those of CIHR more generally.
The IG’s SD is regarded by stakeholders as a leading source of information in the Institute’s mandate areas and that he is well respected in the wider community, both nationally and internationally. He is also seen by stakeholders to be adding credibility to the Institute and defers to experts when relevant.
Under the current SD leadership, IG convened a “Best Brains Exchange” with Health Canada in 2013, to explore the possibility of integrating epigenetic effects of food and environmental contaminants into risk assessments. In 2016, IG also collaborated with the Institute of Aboriginal Health (IAPH) and INMD to organize a ‘DNA on Loan’ conference, which discussed best practices for genetic and biomedical researchers in order to facilitate interaction with Indigenous communities.
Public outreach has been one of IG’s main priorities under the current SD. IG has supported between 10 and 20 national meetings per year. IG organized numerous Café Scientifiques, which provide insight into health-related issues of popular interest. For the past 3 years, Café Scientifiques organized by IG were aired through a partnership with Canal Savoir.
IG also collaborated with CIHR’s Marketing and Communications Branch to co-host the first-ever CIHR Science Writers Workshop, which brought together the Canadian VHO community’s top medical reporters, communicators and genetics researchers.
At the international level, in 2012, IG announced the creation of Orphanet‑Canada, the Canadian branch of Orphanet, the world's online reference portal for rare diseases and orphan drugs, which includes 37 countries. In addition, IG supports the International Rare Diseases Research Consortium (IRDiRC), a global collaborative effort involving over 18 countries established in 2011. IG’s current SD chaired the IRDiRC Executive Committee for three years until December 31, 2015.
Since 2012, IG facilitated Canadian participation in 5 joint transnational calls of E-Rare-3 ERA-NET consortium, which is a key international research partnership that coordinates research activities carried out at the national or regional level in member states.
Partnering to Achieve CIHR and Institute Objectives
Under the leadership of the current SD, IG fosters effective partnerships with all stakeholder groups via reliable collaboration, scientific integrity, and research excellence. The institute has been actively seeking out partnership opportunities and reaching out to various organizations. Partner organizations include other CIHR Institutes, government agencies and departments, and not-for-profit organizations (e.g. health charities). Partnerships are primarily with national organizations, with some collaboration at the international level. A sample of the IG’s partners is found in Appendix 4.
IG’s partnerships and collaborations with other entities took several forms, such as collaborating and convening to enable knowledge exchange and networks of researchers and practitioners, raising more research funding as well as increasing the capacity within specific research areas.
Partnerships have proven to be a significant contributor to funding research under IG mandate over time as depicted in Figure E (Appendix 3). The annual partner contributions to funding opportunities under IG mandate had an overall increasing trend from $3.7 M to $37 M between 2001‑02 and 2015‑16. Between 2010 and 2015, contributions from federal partners (29%) and international organizations (29%) comprised the largest shares of the partners’ contribution to IG mandate. Collaborating with the Government of Canada, IG invested $2.3 M in “Rare Diseases: Models and Mechanisms (RDMM)”, a project supported by the Rare Diseases Research Catalyst Network and facilitates collaboration between basic and clinical scientists in functional studies of novel rare disease genes. Also the CIHR-Genome Canada partnership is one of the most significant public sector investments in personalized medicine. As of 2012, the partnership’s cumulative investments surpassed $165 M, including a combined total of $68.8 M from CIHR, IG, ICR, INMHA, III, INMD and IHSPR.
Contributions from other partners involved, IG co-funding with the Canadian Gene Cure Foundation (CGCF) the “$90,000 Champions of Genetics: Building the Next Generation Grant” in order to enable young scientists advance their genetic research while mentoring the next generation of scientists. Moreover, IG in collaboration with ICR, Genome Canada, the Canadian Agency for Drugs and Technologies in Health (CADTH) and the Axe éthique et santé des populations, sponsored the Canadian GE3LS and Health Services & Policy Research Conference, which examined the integration of new genomic technologies into the healthcare system, at the level of practice and policy. The conference also considered the implications for patients and their families, as well as clinicians, health care systems and society at-large.
4. Operational Effectiveness
Overall, IG is viewed as operating effectively, while wider reformsFootnote 19 at CIHR are viewed less favorably in terms of the resulting impacts.
The Institute is recognized as fostering successful working relationships and the levels of effectiveness, training, and organization are viewed positively. However, additional support and targeted funding was identified as potentially useful. Despite budgetary constraint, IG’s pursuit of its strategic and operational plans has led to progress and the implementation of initiatives in various areas. In contrast, the implementation of reforms at CIHR are perceived as having been undertaken in a sub-optimal manner and are viewed as not particularly beneficial or useful to the Institutes. Additionally, the reforms are seen as resulting in reduced resources, such as funding.
The Institution within which IG operates receives $1 M annually from CIHR as an Institute Support Grant (ISG). An annual average of 54% of the funds was spent on Institute Operations and the remainder was used for Institute Strategic Development. Because IG does not spend all of its ISG funding annually, the balance is transferred to the next fiscal year and therefore the total annual funds available for ISG exceed the $1M allotment to the Institute every year (Figure F, Appendix 3).
V. References
- Canadian Institutes of Health Research, Institute of Genetics, Internal Assessment for 2011 International Review, 2011
- Canadian Institutes of Health Research, CIHR Act
- Canadian Institutes of Health Research, Institute of Genetics, Institute of Genetics Strategic Plan 2012-2017, 2012
- Canadian Institutes of Health Research, Institute of Genetics, Institute of Genetics Strategic Plan 2004-2009, 2005
- Canadian Institutes of Health Research, Institute of Genetics, IG Institute Community Support (ICS) Program 2014‑15, 2014
- Canadian Institutes of Health Research, Personalized Health
- Canadian Institutes of Health Research, Personalized Medicine
- Canadian Institutes of Health Research, Institute of Genetics, 2015–16 Institute Annual Governing Council Update, 2016
- Genome Canada, Request for Applications - 2017 Large-Scale Applied Research Project Competition: Genomics and Precision Health, January 2017
- Canadian Institutes of Health Research, 2007-2008 Departmental Performance Report [ PDF (1.2 MB) - external link ] , 2008
- International Innovation, Beyond your genes [ PDF (694 KB) - external link ], 2014
- Canadian Institutes of Health Research, Institute of Genetics, IG Annual Report to Governing Council 2013-2014
- Alberta Children’s Hospital Research Institute, Business Plan 2015-2020 [ PDF (3.2 MB) - external link ], 2015
- Genes to Genome, Rare disease expert Kim Boycott joins the GENETICS editorial board, 2014
- Canadian Institutes of Health Research, CIHR and Precision Medicine: Personalized Health – CAG Symposium [ PDF (711 KB) - external link ], 2016
- Genome Canada, New pan-Canadian program to accelerate data sharing in biomedical research and patient care, 2015
- Maternity Infant Child & Youth Research Network, Annual Report 2013, 2013
- CQDM, Personalized medicine: CQDM and CIHR fund two promising research collaborations aligning academic and industrial research, 2015
- Orphanet Canada [ PDF (1.5 MB) - external link ]
- Canadian Institutes of Health Research, Institute of Genetics, Rare insight: Research improving lives of people with rare diseases
- International Human Epigenome, Consortium, IHEC Executive Committee
- Austrian-Canadian Science and Innovation Days
- Canadian Institutes of Health Research, Institute of Genetics, The 15th Annual New Principal Investigators Meeting , 2016
- Canadian Institutes of Health Research, Institute of Genetics, Guidebook For New Principal Investigators
- Canal Savoir, Trans-generations: trans history, health, and politics in Montréal and beyond
- Canadian Institutes of Health Research, Internal Audit of the Institute Support Grant
- Canadian Institutes of Health Research, CIHR Initiatives
- Nature, Patient-centric trials for therapeutic development in precision oncology, 2015
- PriceWaterhouse Coopers, The new science of personalized medicine: Translating the promise into practice [ PDF (752 KB) - external link ]
VI. Appendices
Appendix 1: IG Review Panel Members’ Affiliations and Conflict of Interest Declaration
Chair:
- Daniel Durocher, Senior Investigator, Lunenfeld-Tanenbaum Research Institute
Panel Members:
- Bartha Knoppers, Professor and Director, Centre of Genomics and Policy, McGill University, Canada
- Monica Justice, Professor of Molecular Genetics, Program Head and Senior Scientist, University of Toronto, Canada
- Durhane Wong Rieger, President and CEO of the Canadian Organization for Rare Disorders, Canada
- Han Brunner, Professor and Head of Clinical Genetics, Maastricht UMC+, Netherlands
- David Valle, Director, Institute of Genetic Medicine, Johns Hopkins University, US
| Panel Member | Conflict of Interest Declaration |
|---|---|
| Daniel Durocher | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
| Bartha Knoppers | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
| Monica Justice | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
| Durhane Wong Rieger | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
| Han Brunner | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
| David Valle | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Review Panel |
Appendix 2: Overview of Data Sources and Methods
| Data source | Description |
|---|---|
| Situational Analysis (SA) |
|
| Key informant interviews |
|
| Bibliometric Analysis |
|
Appendix 3: Key Figures and Tables
- Figure A : CIHR Investment in IG Mandate by 2012-17 Research Priorities
- Figure B: Investment in Capacity Building out of IG Budget
- Figure C: Percentage of Direct Trainees Funded under IG Mandate
- Figure D: Percentage of Indirect Trainees Supported under IG Mandate
- Figure E: Leverage Ratio of Partnership to CIHR Investment in IG Mandate
- Figure F: Utilization of Institute Support Grant (ISG) Budget
Figure A: CIHR Investment in IG Mandate by 2012-17 Research Priorities
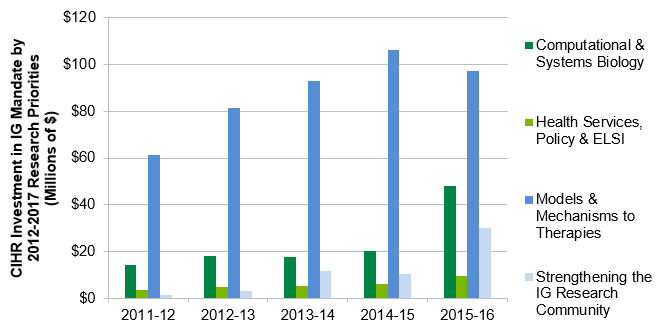
Figure A – Long description
| 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|
| Computational & Systems Biology | $14,252,783 | $18,051,263 | $17,799,063 | $20,280,618 | $48,275,934 |
| Health Services, Policy & ELSI | $3,433,643 | $4,652,108 | $5,236,045 | $5,944,252 | $9,629,936 |
| Models & Mechanisms to Therapies | $61,540,341 | $81,599,683 | $93,055,561 | $106,246,940 | $97,213,512 |
| Strengthening the IG Research Community | $1,285,170 | $3,281,226 | $11,755,509 | $10,436,319 | $29,965,960 |
Figure B: Investment in Capacity Building out of IG Budget
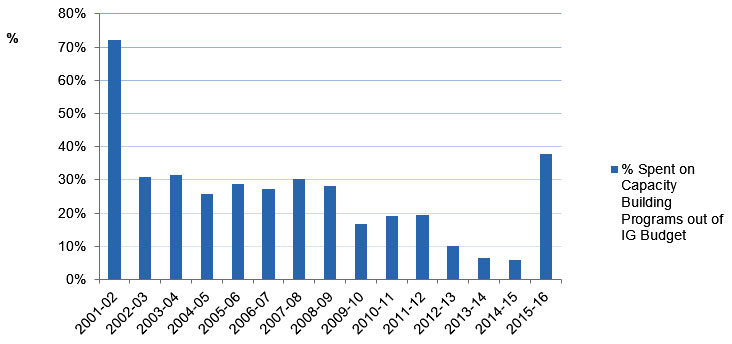
Figure B – Long description
| 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Spent on Capacity Building Programs out of IG Budget | 72% | 31% | 32% | 26% | 29% | 27% | 30% | 28% | 17% | 19% | 19% | 10% | 6% | 6% | 38% |
- IG’s spending on Capacity building programs as a percentage of IG’s annual budget had an overall decreasing trend (decreasing from 72% in 2001-2002 to 6% in 2014‑15.
- It is worth noting that the percentage invested in capacity building in 2015‑16 is high not due to an actual increase in the money spent but due to a drop in the institute Strategic budget to $1 M because of commitments to the RAF.
Figure C: Percentage of Direct Trainees Funded under IG Mandate
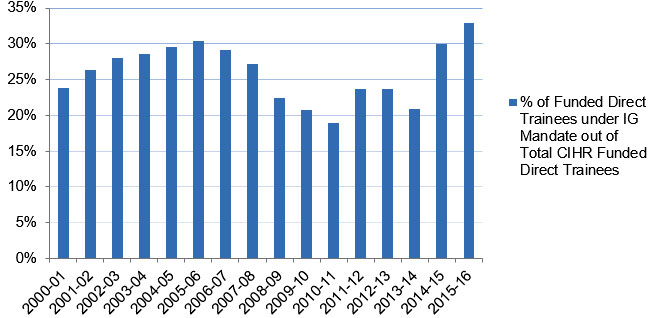
Figure C – Long description
| 2000‑01 | 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Funded Direct Trainees under IG Mandate out of Total CIHR Funded Direct Trainees | 24% | 26% | 28% | 29% | 30% | 30% | 29% | 27% | 22% | 21% | 19% | 24% | 24% | 21% | 30% | 33% |
- The annual number of Direct Trainees funded under IG’s mandate expanded from 370 in 2000‑01 to approximately 600 in 2006‑07. The figure surpassed 730 in 2014‑15 and 2015‑16.
- The number of Direct Trainees funded under IG’s mandate, as a proportion of the total CIHR-funded direct trainees, ranged from 24% to 30% between 2000‑01 and 2007‑08. The minimum share (19%) was reached in 2010‑11, while the maximum (33%) was achieved in 2015‑16.
Figure D: Percentage of Indirect Trainees Supported under IG Mandate
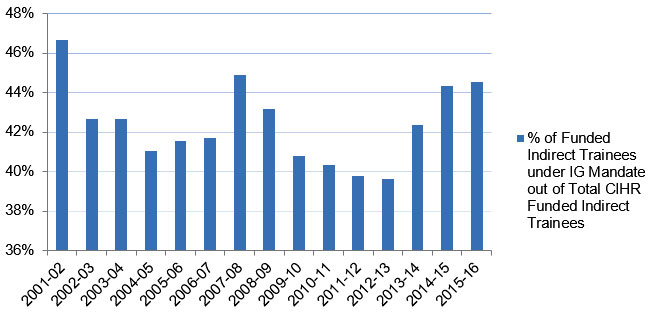
Figure D – Long description
| 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Funded Indirect Trainees under IG Mandate out of Total CIHR Funded Indirect Trainees | 47% | 43% | 43% | 41% | 42% | 42% | 45% | 43% | 41% | 40% | 40% | 40% | 42% | 44% | 45% |
- The number of Indirect Trainees funded under IG’s mandate increased from 2001‑02’s 1,142 to 2,632 in 2006‑07. The figure fell to roughly 2,600 during the next couple of years, and then experienced an uptick, ultimately reaching its maximum of 3,151 in 2013‑14 before it fell again in 2015‑16.
- The average annual percentage of the Indirect Trainees funded under IG’s mandate out of the total number of those funded by CIHR was 42% over the past 15 years.
Figure E : Leverage Ratio of Partnership to CIHR Investment in IG Mandate
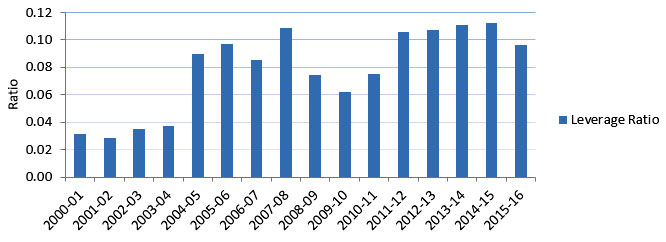
Figure E – Long description
| 2000‑01 | 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leverage Ratio | 0.03 | 0.03 | 0.03 | 0.04 | 0.09 | 0.10 | 0.09 | 0.11 | 0.07 | 0.06 | 0.08 | 0.11 | 0.11 | 0.11 | 0.11 | 0.10 |
- The leverage ratio of partnership to CIHR investment shows how much was invested in IG mandate through partner contributions for every dollar of CIHR investment in the IG’s mandate areas. This ratio had an overall increasing trend from 0.03 to 0.11 (between 2000‑01 and 2007‑08). The ratio then dropped until it reached 0.08 in 2010‑11 after which it rose to 0.11 and was maintained to 2014‑15 before it slightly decreased to 0.10 in 2015‑16.
Figure F: Utilization of Institute Support Grant (ISG) Budget
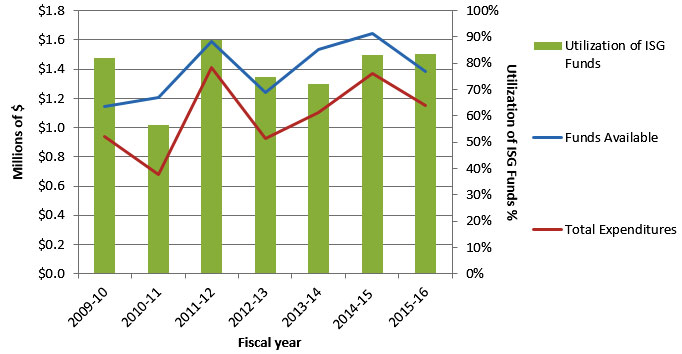
Figure F – Long description
| 2011‑12 | 2012‑13 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|
| Total Funds Available for Current Year | $1,145,580 | $1,204,961 | $1,587,559 | $1,237,348 | $1,536,687 | $1,643,888 | $1,380,629 |
| Total Expenditures | $940,619 | $680,159 | $1,408,084 | $925,632 | $1,106,074 | $1,369,215 | $1,152,185 |
| Utilization of ISG Funds | 82% | 57% | 88% | 75% | 72% | 84% | 83% |
- The unspent portion of the annual Institute Support Grant (ISG) is transferred to the next fiscal year’s budget. Consequently, as the above chart indicates, the “Funds Available in $M” exceeds the $1M of ISG funds provided by CIHR each year.
- From 2009‑10 to 2015‑16, IG annually spent as little as 57% (2010‑11) and as much as 88% (2011‑12) of the ISG funds at its disposal.
- Given its sound management of its funds, IG will be able to ensure a 6-months period with the Montréal staff assisting the establishment of the new team and their introduction in the various networks developed under Paul’s current mandate. Expected balance at the end of this period is of $20,000, which represents 0.25% of the overall $8M budget managed over 8 years.
Appendix 4: Sample list of Partners
- National Research Council (NRC);
- Natural Sciences and Engineering Research Council (NSERC);
- Genome Canada;
- Health Canada;
- CIHR Institutes;
- Heart & Stroke Foundation;
- Cystic Fibrosis Foundation of Canada;
- Muscular Dystrophy Canada;
- Fonds de recherche du Québec;
- Kidney Foundation of Canada;
- Canadian Gene Cure Foundation (CGCF);
- Maternal Infant Child & Youth Research Network (MICYRN);
- Ataxia of Charlevoix-Saguenay Foundation;
- University of Toronto;
- Mount Sinai Hospital’s Lunenfeld-Tanenbaum Research Institute;
- Canadian Agency for Drugs and Technologies in Health (CADTH);
- Axe éthique et santé des populations;
- Canadian Agency for Drugs and Technologies in Health (CADTH);
- Japan Science and Technology Agency (JST);
- French National Research Agency (ANR);
- German Federal Ministry of Education and Research (BMBF);
- European Commission;
- International Rare Diseases Research Consortium (IRDiRC);
- International Human Epigenome Consortium (IHEC);
- Structural Genomics Consortium (SGC);
- Autism Genome Project.
- Date modified: