Review of the Institute of Infection and Immunity (III)
Report of the III Review Panel
March 2018
At the Canadian Institutes of Health Research (CIHR), we know that research has the power to change lives. As Canada's health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
Canadian Institutes of Health Research
160 Elgin Street, 9th Floor
Address Locator 4809A
Ottawa, Ontario K1A 0W9
This publication was produced by the Canadian Institutes of Health Research. The views expressed herein do not necessarily reflect those of the Canadian Institutes of Health Research.
III Review Panel:
Chair: B. Brett Finlay, Professor, Microbiology and Biochemistry, Michael Smith Laboratories, University of British Columbia
Panel Members:
- Megan Levings, Professor, Department of Surgery, University of British Columbia;
- Allison McGeer, Professor, Faculty of Laboratory Medicine & Pathobiology; Dalla Lana School of Public Health, University of Toronto;
- Claude Perreault, Professor, Department of Medicine, University of Montreal;
Thanks to all participants in this review and the CIHR III Review Team: Ian Raskin, Michael Goodyer, Doaa Saddek, Kim Gaudreau, Christopher Manuel, Jonathan Gilbert, Jean‑Christian Maillet, Sheldon Polowin, and Carole Chow.
And special thanks to: Dr. Marc Ouellette, Scientific Director, III, Élisabeth Pagé, Assistant Director, III, and the III team.
For more information and to obtain copies, please contact: Evaluation@cihr-irsc.gc.ca.
Table of Contents
- Executive Summary
- Overview of the Review and III
- Observations and Recommendations
- Review Key Findings
- References
- Appendices
I. Executive Summary
The review of the Institute of Infection and Immunity (III) was undertaken by the Canadian Institutes of Health Research (CIHR) as part of the review of the mandate and performance of CIHR Institutes by CIHR’s Governing Council (GC) outlined in the CIHR Act. The review assessed the relevance and performance of III to inform decisions regarding the role and functioning of the Institute. The review was conducted by the CIHR Evaluation Unit and overseen the III Review Panel (hereafter referred to as the Panel) – a panel of experts in III’s mandate areas who reviewed and interpreted the findings and made the final recommendations. The observations and recommendations of the Panel are summarized below in relation to the two broad issues addressed by the review.
Are changes needed within the current III mandate in order to address emerging areas of research?
The Panel concludes that III’s mandate is appropriate given the fact that it is very broad and all encompassing across many diseases. The Panel noted that the diseases touched by infection and immunity are also addressed by the mandate of the other CIHR Institutes (e.g. diabetes, heart disease and cancer), which speaks to the importance of collaboration between III and the other CIHR Institutes.
Recommendation 1: The Panel recommends that III continue with the current mandate.
Going forward, the Panel sees a need for III to continue to lead in the areas of emerging threats, notably antimicrobial resistance (AMR), and HIV/AIDS, as well as any new infectious threats that will arise. That said, the Panel highlights that, in comparison to the area of immunology, the areas of emerging threats, HIV and AMR have received more attention and investment as Government of Canada strategic priorities.
Recommendation 2: The Panel recommends that III continue to lead on emerging threats, AMR, and HIV/AIDS initiatives.
The Panel understands, however, that maintaining the balance between infection and immunity is a difficult and perennial challenge and will continue to pose a challenge for the next SD. The next SD needs to consider an Assistant Director with an area of expertise that complements that of the SD to help strike a balance between the two mandate areas. In addition, it will be important to ensure there is a balance between the infection and immunity experts on the new Institute Advisory Board (IAB).
Recommendation 3: The Panel recommends hiring an Assistant Director, whose area of expertise complements that of the next SD.
Observations and Recommendations for the Next Scientific Director
As the current SD of III will complete his second and final term in June 2018, the Panel provides advice to GC and CIHR to inform the transition of the Institute to the next SD. The current III SD has demonstrated strong skills in engaging the research community and is well-regarded by both researchers and stakeholders. It will be important for the next SD to build on the successful initiatives and partnerships of the current SD and establish new innovative partnerships domestically and internationally. In order to help ensure the necessary support as well as maintain a balance of experts in infection and immunity, it is important that the next SD have the opportunity to select some of the members of the new IAB.
Recommendation 4: The Panel recommends that the next SD have the opportunity to select some of the members of the new IAB.
The Panel observes that unless researchers have participated in Institute organized workshops or received strategic funding, they tend to be unaware of the Institute’s activities. As a result, it is advisable that the next SD work on establishing an effective outreach and communication strategy that reaches the full III mandate. The Panel notes that mid-career investigators are currently under supported and recommends that the next SD target the communication and outreach strategy to mid-career investigators to raise awareness of III’s role and funding mechanisms and facilitate discussion around creating innovative funding structures.
Community building activities such as travel awards, studentships, workshops that focus on emerging areas (e.g. microbiome, immunotherapies) could help improve the Institute’s outreach to the community. Here, the Institute can build on the success of the New Investigator Forum model to target mid-career investigators.
Recommendation 5: The Panel recommends the next SD target communication and outreach activities to mid-career investigators to raise awareness of III’s role and funding mechanisms, and facilitate discussion around creating innovative funding structures.
As part of the transition of the Institute, the Panel sees a need for a formalized process to facilitate the transition between SDs. For example, the outgoing SD and incoming SD should meet to discuss the transition of Institute priorities and operations as well as key initiatives and collaborations. Related to this, the Panel feels it will be important for the next SD to strike a balance between the needs of preparing to react to emerging threats, which is unique to III mandate, and establishing and investing in the priorities of the next strategic plan.
Recommendation 6: The Panel recommends that the next SD, as part of the strategic planning process, assess previous funding opportunities to identify, prioritize, and target future funding opportunities.
II. Overview of the Review and III
A. Review Objectives
The review of the III was conducted by CIHR as part of the rolling review of the mandate and performance of the 13 CIHR Institutes. The review assessed the relevance and performance of III to inform future direction and focus of its mandate. The aim of the review is to provide the GC with findings to inform decisions to:
- Provide CIHR management with valid, insightful, and useful findings regarding the ongoing institute relevance and performance; and
- Inform decisions regarding the transition of the Institute and the next SD.
The review was overseen by the III Review Panel comprised of experts in the III mandate areas who reviewed and interpreted the findings and made the final recommendations. The names and affiliations of the Panel members are listed in Appendix 1. The review was conducted by the CIHR Evaluation Unit.
The review covered the period 2000-2017, with a focus on the period under the leadership of the current SD, Dr. Marc Ouellette, from 2010 and 2017Footnote 1. Using a common framework of analysis, the review drew on multiple lines of evidence, including qualitative and quantitative data sources outlined in Appendix 2 with key figures presented in Appendix 3. The review used administrative data on expenditures related to the III mandate, bibliometric analysis on the ranking of Canada compared to the top active countries in the fields of infection and immunity research, interviews with a number of III researchers and stakeholder representatives and Panel deliberations. While each line of evidence has limitations, there is convergence among them so as to produce key findings. Overall, we are reasonably confident that the results presented provide an accurate portrait of the relevance of III’s mandate and the Institute’s performance.
B. CIHR Context and the Canadian Funding Landscape
As outlined in the CIHR Act, the objective of the CIHR is:
“to excel, according to internationally accepted standards of scientific excellence, in the creation of new knowledge and its translation into improved health for Canadians, more effective health services and products and a strengthened Canadian health care system…”
Among the many activities to achieve its objective, CIHR is responsible for “encouraging innovation, facilitating the commercialization of health research in Canada and promoting economic development through health research in Canada.” And, as divisions within CIHR, the Institutes are expected to contribute to the achievement of CIHR’s overall objective within their mandate through a number of activities, including: “work in collaboration with the provinces to advance health research and to promote the dissemination and application of new research knowledge to improve health and health services.”
The Government of Canada’s Budget 2018 provides unprecedented support for fundamental research through the three federal granting agenciesFootnote 2. For CIHR, this results in an increase to CIHR’s budget of $354.7 M over 5 years starting in 2018-19. This represents an ongoing investment of $90.1 M per year for investigator-led research. The 2018 Budget responds to the Canada's Fundamental Science Review, released in 2017, that stressed the need for significant reinvestment in the federal research ecosystem over a more predictable and better planned multi-year horizon.Footnote 3 Prior to Budget 2018, CIHR’s annual budget had remained relatively stable for approximately the last 10 years, and therefore it was declining substantially in real terms, greatly affecting the ability of researchers to sustain competitive research programs. The majority of CIHR funding of research under the mandates of the 13 Institutes is investigator-initiated research fundingFootnote 4; whereas the Institutes’ strategic research budgets, which are comparably smaller, are used to play a role of catalyst with strategically placed investmentsFootnote 5.
CIHR is composed of 13 institutes and each of them received a strategic research budget of $8.6 M until 2014-15. As a result of the Institute Modernization, in 2015-16, half of each Institutes’ strategic research budgets ($4.3 M per year) was invested in CIHR’s Roadmap Accelerator Fund (RAF) to support multi-Institute and multidisciplinary initiatives align with CIHR’s research priorities patterned along the lines of existing CIHR Initiatives. The remaining half of the budget remains under the control of Institutes to direct toward Institute-specific initiatives.Footnote 6 As of 2017-18, Institutes returned to a strategic research budget ($8.6 M); however, the investments of funds in multi-Institute and multidisciplinary initiatives are guided by the same “spirit” and principles as RAF.
CIHR’s structure and approach to the staff allocated to support the 13 institutes has changed in recent years. Before 2014-15, there were Ottawa-based Institute staff (OBIS), the personnel at CIHR’s central office dedicated to provide service to each of the 13 Institutes. After that, the staff allocation model changed to Institute based staff, working in Integrated Institute Teams (IIT) that provide support across all 13 Institutes. This change created pressure on the Institute operating budget to cover staffing costs that were previously covered by CIHR’s central operations.
C. III Scientific Landscape
The Panel observes that the following research areas are key priority areas within the fields of infection and immunity in the year to come:
- The area of the microbiome is growing rapidly and affects many health and disease areas within CIHR’s mandate. The microbiome also provides a strong link to immunology, including immune therapy, cancer therapy, and many other areas. III is well centered to embrace the microbiome, and it provides a strong link between microbiology and immunology.
- Systems immunology is also rapidly expanding, and overlaps to some extent with both infectious agents and the microbiome.
- Other priority areas include sex and gender, aging (chronic inflammation and the role of the microbiome in this), immunocardiology, health inequities, immune cell engineering, and stem cell transplants.
- Maintaining and building on investments in vaccines and novel adjuvants is a gap in the Canadian research landscape as well as the absence of vaccine evaluation.
- AMR is the 21st century crisis, which encompasses both infections and the microbiome.
D. III Context
As one of the 13 CIHR Institutes, III has a vision to become the Canadian focal point of reference to harness and optimize the research efforts in infectious and immune-related health and disease. III aims to become the national and international reference in the utilization and implementation of those research results for the improvement of the health care system.Footnote 7
III’s mandate is to support research to enhance immune-mediated health and to reduce the burden of infectious disease, immune-mediated disease and allergy through prevention strategies, screening, diagnosis, treatment, support systems and palliation. The mandate transcends disciplines and encompasses all four CIHR health research themesFootnote 8. The unique challenge that III faces, given the nature of its mandate, is for the Institute to be reactive and responsive to emerging threats. Within its mandate, III established two main strategic objectives to shape the Institute activities and research priorities:
- Strengthening and coordinating infection and immunity research; and
- Facilitating the application and impact of research.Footnote 9
III’s current SD has demonstrated success, particularly in a wide range of health concerns, including AMR, the microbiome, hepatitis C, HIV/AIDS, pandemic influenza, transplantation, inflammation in chronic disease and vaccine technologies. III manages and oversees the research component of the Government of Canada HIV/AIDS Initiative, which provides approximately $21 M annually to support researchers and trainees. The Institute also cooperates and works together with CIHR’s 12 other institutes on several initiatives. For example, III co-leads the Environments and Health Initiative, with the Institute of Population and Public Health (IPPH); and the initiative on Inflammation in Chronic Disease, with the Institute of Musculoskeletal Health and Arthritis (IMHA). III also works closely with IPPH on antimicrobial resistance and Ebola and with the Institute of Circulatory and Respiratory Health (ICRH) on the area of transplantation.
III is committed to and supports capacity building through partnerships with other health organizations and professional associations, as well as by providing support to new investigators though holding the New Investigator Forum (NIF) biennial meetings that provide networking opportunities, career advices and grant review tips to new investigators.
Although III returned to an $8.6 M strategic research budget in 2017-18, it will have limited impact on the research fund available for the next SD due to forward commitments to CIHR and Institute initiatives. For example, based on the most recent budget and commitments, the next SD will have a limited available or uncommitted research budget for much of their first four-year term: 2018-19 ($4 K), 2019-20 ($1.57 M); 2020-21 ($2.44 M); and 2021-22 ($4.4 M)Footnote 10. The limited unencumbered budget available for the next SD may hamper his/her ability to invest in the III’s new priorities for first few years of his/her tenure.
The largest percentage of total CIHR investment in the III mandate research area originates mainly from investigator-led operating grants competitions, which are not managed by the III SD. The total CIHR investment in III mandateFootnote 11 increased between 2000-01 and 2010-11, from $97 M to $282 M. It then decreased slightly until 2013-2014 before it increased to $292 M in 2016-17. For more information about CIHR investments in III’s mandate by research priorities (see Appendix 3).
In response to the restructuring of OBIS from providing services to one Institute to providing specific management expertise across Institutes, III hired additional Institute-based staff. The Institute started with three staff members and over time reached five. The Institute based staff work on the development of the RFAs and the scientific component of the Institute’s work. The Institute was able to cover the increase in costs due to staffing as it depended on the unspent operational budget from the previous SD. However, III is still in need for additional administrative staff and the current annual $1 M Institute Support Grant (ISG) that the Institute receives in support for operations is not enough to support further hiring of staff.
III. Observations and Recommendations
A. Are changes needed within the current III mandate to address emerging areas of research?
1. Panel Observations
The Panel noted that some of the diseases under III’s mandate also reside under the mandate of the other CIHR Institutes (e.g. diabetes, heart disease, cancer), which enables collaboration and partnership between III and the other CIHR Institutes. The Panel highlighted that when compared to the area of immunology, the areas that have received more attention under the III mandate so far are: emerging threats, HIV/AIDS and AMR. It is important to note that these areas represent Government of Canada strategic priorities with dedicated funding that is separate from III’s strategic research budget. From 2010-11 to 2016-17, III investments in infection and immunity were equally distributed. The Panel noted that currently, most of III mandate funding goes to basic research and it should remain this way, since the role of CIHR is to occupy this niche.
The Panel sees that maintaining the balance between infection and immunity areas is difficult to accomplish and will pose a challenge to the next SD. The Panel, therefore, suggests that having an Assistant Director, whose area of expertise complements that of the next SD, and could provide the necessary support to the SD in striking the balance between the two mandate areas of infection/microbiology and immunology. Further, it is suggested to maintain a balance between the infection and Immunity experts on the new IAB.
2. Recommendations
Recommendation 1: The Panel recommends that III continue with its current mandate.
Recommendation 2: The Panel recommends that III continue to lead on emerging threats, AMR, and HIV/AIDS initiatives.
Recommendation 3: The Panel recommends hiring an Assistant Director, whose area of expertise complements that of the next SD.
B. Observations for the Next Scientific Director
1. Panel Observations
There is widespread appreciation for the current SD in the III research and stakeholder community. III’s current SD has been a champion of AMR and HIV/AIDS research has that led Canada to play a leadership role in these two areas. The next SD should sustain the established partnerships and he/she should promote and create new and innovative partnerships, domestically and internationally.
The return of the Institute-specific IAB will be important for the next SD to help ensure he/she has the domain expertise required to develop and focus on strategic priorities, and to obtain advice from and communicate with its research community. The Panel understands that the current SD has already started recruiting new IAB members, but it is advised that some of the members of the IAB be left to the discretion of the next SD. It is also advisable that the current SD be part of the transition and orientation process of the next SD in order to help ensure a smooth transition.
The next SD needs to have a vision and the ability to act quickly on that vision. The next SD needs to create a balance between proactive and reactive funding opportunities to be able to manoeuver and to quickly respond to emerging areas. In consideration with expertise, III needs to have a core body of researchers across Canada, ready to be mobilized. III has been providing opportunities for the mentorship of new investigators through the New Investigator Forum. The Panel noted that it is advisable that the next SD work on establishing an effective outreach and communication strategy that reaches the full spectrum of researchers working under the broad III mandate, especially one that targets mid-career investigators.The next SD should also reach out to the community via organizing activities (e.g. workshops) that focus on emerging areas (e.g. microbiome and immunotherapies).
According to the researchers’ interviewed, the bridge funding Footnote 12 is valued and it should be maintained. The Panel highlighted that the next SD should start his/her tenure by conducting an assessment of the success and effectiveness of the past RFAs as this would enable him/her to address gaps and focus the priorities in the next strategic plan.
2. Recommendations
Recommendation 4: The Panel recommends that the next SD have the opportunity to select some of the members of the new IAB.
Recommendation 5: The Panel recommends the next SD target communication and outreach activities to mid-career investigators to raise awareness of III’s role and funding mechanisms, and facilitate discussion around creating innovative funding structures.
Recommendation 6: The Panel recommends that the next SD, as part of the strategic planning process, assess previous funding opportunities to identify, prioritize, and target future funding opportunities.
IV. Key Findings
A. Relevance
1. Ongoing relevance of the III mandate
The III 2013-2018 Strategic Plan focuses on two priorities: chronic disease prevention; and emerging threats. From 2013-14 to 2017-18, the amount invested by CIHR in chronic disease prevention represents an annual average of 64% of total CIHR investment in III mandate, compared to 36% in emerging threats. Appendix 3 presents CIHR’s investment in III mandate by the Institute’s research priority areas, as defined in its 2013-18 Strategic Plan. The previous strategic plan (2007-2012) had five different priorities: HIV/AIDS; Emerging Infections & Microbial Resistance, Immunotherapy, Vaccines, Pandemic Influenza preparedness. Between 2007-08 and 2011‑12 the largest CIHR investment in III mandate was in the HIV/AIDS research priority with an annual average of 31% compared to 30% in emerging infections & microbial resistance.
III investment in CIHR Initiatives out of its own budget increased from 25% in 2011-12 to 63% in 2014-15. Over the period from 2011-12 to 2016-17, III contributed financially to 6 out of CIHR’s 22 current major initiativesFootnote 13. These are: Antimicrobial Resistance, Collaborative Health Research Project (CHRP), HIV/AIDS, Inflammation in Chronic Disease, Pandemic Preparedness, and Personalized Medicine/Health. The highest contribution was in Inflammation in Chronic Disease, 28% of III’s budget over this 6 year period.
The bibliometric analysisFootnote 14 shows that between 2011 and 2016, Canada’s ranking decreased from the 7th to 8th concerning the number of publicationsFootnote 15 in the six priority areas combined, accounting for an average of 4% of the world’s total annual publications. Results show that Canadian researchers are publishing in journals that are cited more often than the world average. Between 2011 and 2016, the specialized index (SI)Footnote 16 shows that Canada is more specialized in the HIV/AIDS, Drug resistance and Infections Agents priority areas compared to the world average. However, Canada is slightly less specialized in the Inflammation, Transplantation and Vaccine priority areas. Also, countries such as the Netherlands, US, Italy and Spain have consistently had higher SI than Canada over time. From 2000 to 2016, on average 46% of Canadian annual publications in III’s priority areas were co-authored with researchers from another country.
III has been playing an integral role in developing, promoting and maintaining a research community that addresses issues related to infections and immunity. Infection and immunity research priorities are supported financially by CIHR more broadly and by III specifically. The research community members interviewed generally agreed that III has managed to meet its mandate, especially considering the broadness of the mandate and the limited funds available. The current SD was recognized by the researchers for his ability to leverage funding, to build capacity. Although, some members of the research community thought that the Institute has focused too narrowly on HIV/AIDS and Hepatitis C and coverage of other areas would be needed as well. The stakeholder representatives interviewed noted that III partnered effectively to strengthen and coordinate infection and immunity research in Canada and abroad.
B. Impact
1. Support to Innovative Research and Advancing Knowledge
The outgoing SD has been actively working toward supporting innovative research and advancing knowledge. Through III’s efforts, CIHR has developed many research initiatives, such as AMR, tuberculosis, Zika, Ebola, HIV/AIDS that address global health challenges
In the fall of 2014, CIHR launched the Ebola Research Funding Initiatives to support research that sought to develop new approaches to treating, preventing and containing the diseaseFootnote 17. III funded Ebola projects include:
- Innovative Ebola Research;
- Ebola Vaccine Rapid Response – Cell-mediated Immunity; and,
- Interferon for Ebola Treatment and the Ebola Vaccine Phase II/III Clinical Trial.
III has also partnered with the Public Health Agency of Canada (PHAC), International Development Research Centre (IDRC), Global Affairs Canada (GAC) and various other Government of Canada departments on various Ebola-related initiatives. Overall, the work done by III in the field of Ebola research has triggered the creation of guidelines for the treatment of the disease. The research community and the stakeholder representatives interviewed flagged the development of the Ebola vaccine as a great example of innovative research, and knowledge translation that the III supported.
III work has also helped governments address specific and immediate questions such as the value of Ontario’s universal seasonal influenza immunization program and ways to effectively address C. difficile infections.
Moreover, III funded several innovative research projects that led to the creation of new knowledge under the area of inflammation. The following are examples of the outcomes from III funded research projects.
- The discovery of a new molecule that prevents inflammation and also metastasis to the lung, which has led to a filed patent for a compound that blocks lung cancer and lung inflammation.
- The development of diagnostic biomarkers and identification of key pathways of injury that will result in a pilot therapeutic trial for patients with contrast-induced acute kidney injury (CI-AKI) by Dana Philpott’s team, in 2017.
Some of the researchers interviewed mentioned that III made a great effort in supporting research in translating the knowledge created; however, they highlighted that the translational component of research in general is challenging to achieve because it requires extensive resources and expertise, which are beyond the resources and capacity of the Institute. In the opinion of some researchers and stakeholders, supporting basic science, which is fundamental in III mandate need to accompanied with an effective translational component.
2. Contributions to Building Capacity of the Health Research Enterprise
From 2010 to 2016, III spent an average of 16% of its budget annually on capacity building, including investments in catalyst/pilot programs, training grants and award, and development grants (see Footnote 18 and 28% of indirect traineesFootnote 19 were funded under the III mandate (see Appendix 3).
Since 2014, III participated in multiple calls of the Joint Programming Initiative on Antibiotic Resistance (JPIAMR). Those international calls provide Canadian investigators an opportunity to work with colleagues to build international collaborations, multilateral research projects based on complementarities and sharing of expertise. III led the JPIAMR Working Group on the Virtual Research Institute (VRI) in November 2017 that addresses a wide range of AMR priorities. In January 2016, III participated in the Transmission Dynamics Call for Proposals of the JPIAMR, which involved 20 countries, and provided Canadian investigators an opportunity to work with colleagues to build interdisciplinary, multilateral research projects based on complementarities and share expertise.
Under III’s leadership, the CIHR HIV/AIDS and STBBI Research Initiative invest approximately $21M annually to support research, capacity building and knowledge translations activities.Footnote 20 III provides research training and mentorship environment to support capacity building by prioritizing learning opportunities for students as well as community members and researchers.Footnote 21
III convenes a biennial New Investigator Forum (NFI) to train the next generation of researchers. The NFI features workshops and presentations of information, tools and mechanisms which support career development in research in Canada.Footnote 22 III held 4 fora between 2010 and 2017. In addition, III has developed several funding programs under the Institute Community Support (ICS)Footnote 23 Program to encourage excellence in research and foster community development.
III engages in a number of capacity building activities, including Institute-facilitated capacity building events and other investments to maintain and strengthen research capacity in mandate areas; however, some of the members of the research community interviewed mentioned that III needs to work more on the development of opportunities for knowledge and capacity building, in collaboration with the stakeholder community, are these opportunities are perceived to not be happening as often they should be. According to some interviewees, there is a gap that still needs to be addressed in supporting mid-career investigators.
C. Convener and Catalyst
1. Contribution of Scientific Leadership to the Convener-Catalyst Role
Between 2010 and 2018, III hosted multiple workshops, during which future research agendas were set, research capacity assessed, opportunities for international collaboration defined, and potential partners engaged for future funding opportunities.
The current SD is a vice-chair of the Global Research Collaboration for Infectious Disease Preparedness (GloPID-R) a network of major research funding organizations working on a global scale to facilitate an effective research response within 48 hours of an infectious disease outbreak. The current SD also sits on the management board and the steering committee of JPIAMR, which has the mission to join forces across nations by leading the alignment, coordination, and support to antimicrobial resistance. At the international level, in 2016, the SD was a co‑chair of one of the workshops on the Ebola virus in Accra, Ghana. In 2013, III also organized a workshop with the UK Health Protection Agency (now a part of Public Health England) that brought together UK and Canadian researchers with representatives from industry to encourage the translation of research outcomes into application.
Due to the impacts of high-profile issues such as HIV/AIDS, SARS, Porcine Influenza (H1N1), Ebola, and AMR, media coverage of III has been prominent. The current SD is frequently interviewed by the media and represents CIHR at numerous public events, in reference to the many health issues that fall under III’s purview.
Under the current SD, public outreach has consistently been one of III’s main areas of focus. However, some of the stakeholders interviewed highlighted that the communication with the research community needs further improvement, as the profile and mandate of the Institute seems to be mainly known to the previous grant recipients and the regular workshops and conferences participants. They added that many members of the community are still unaware of the Institute’s activities.
2. Partnering to Achieve CIHR and Institute Objectives
III partnerships and collaborations with other entities took several forms, such as collaborating and convening to enable knowledge exchange and networks of researchers and practitioners, raising more research funding as well as increasing the capacity within specific areas. Partner organizations include all the other CIHR Institutes, government agencies and departments, international partners and not-for-profit organizations, such as health charities (see Appendix 4).
The institute has been actively seeking out partnership opportunities and reaching out to various organizations. Findings show that partnerships have proven to be a significant contributor to funding research under III mandate over time (see Appendix 3). The annual partner contributions to funding opportunities under III mandate had an overall increasing trend from $11 M to $40 M between 2001-02 and 2015-16. Between 2009 and 2014, contributions from federal partners (37%) and international partners (21%) comprised the largest shares of the partners’ contribution to III mandate. From 2014 to 2016, the largest shares of the partners’ contribution to III mandate originated from international partners (36%) and not-for-profit organizations (28%). III collaborates with the Canadian Blood Services (CBS), the Canadian Liver Foundation, Cystic Fibrosis Canada, Genome BC, the Fonds de recherche du Québec – Santé, the Kidney Foundation of Canada, and four other Institutes and CIHR Ethics Office in the Canadian National Transplant Research Program. III partnered with the Bill and Melinda Gates Foundation (BMGF) which resulted in the launch of a joint funding opportunity in the area of mucosal immunology for HIV vaccine development. Overall, the stakeholder representatives and the research community interviewed agreed that III has been excellent in establishing and maintaining partnerships domestically and internationally.
D. Operational Effectiveness
The Institution within which III operates receives $1 M annually from CIHR as an Institute Support Grant (ISG). Before 2011‑12, III did not spend all of its ISG funding annually, the balance was transferred to the following fiscal year and therefore the total annual funds available for ISG exceed the $1M allotment to the Institute every year (see Appendix 3). From 2011-12 to 2015-16, III started to use part of the accumulated surplus, this is due to the number of Strategic Initiatives in which III is involved, the broadness of the mandate and the reallocation of the OBIS, which meant that the Institute had to spend from the accumulated surplus to hire additional Institute staff. Between 2010 and 2015-16, III spent an annual average of 74% on Institute Operations (which include staff salary) and the remainder was used for Institute Strategic Development.
Additional support and targeted funding was identified as potentially useful for the Institute’s operations. Despite budgetary constraint, III’s pursuit of its strategic and operational plans has led to progress and the implementation of initiatives in various areas. Additionally, the current approach that replaced OBIS to support the Institute poses a challenge for III considering the number of initiative under its responsibility and that the current ISG budget is not sufficient to support the required staff.
E. Conclusion
Overall, III met its mandate despite its breadth and the limited funds available. III plays an integral role in developing, promoting and maintain a research environment that addresses issues related to Infections and Immunity. However, it was noted that the III focused mainly on the areas of emerging threats, HIV/AIDS and AMR, and, accordingly, more attention needs to be given to the area of immunology, including emerging areas such as immune therapy and stem cell transplants. The microbiome also has to be incorporated into the mandate, as it overlaps extensively with both infections and immunology, and can help anchor the institute’s mandate, as well as impact in many other areas of health and disease
Through III’s efforts, CIHR has developed many research initiatives that address global health challenges (AMR, tuberculosis, Zika, and Ebola). The development of the Ebola vaccine is an example of the III effort to support innovative research and knowledge translation. Despite the efforts made to support the translation of research, balancing the effort between supporting basic science and knowledge translation remains a challenge for III.
Over the years, III engaged in a number of capacity building activities to maintain and strengthen research capacity in its mandate areas. There is still a need however for innovative techniques to address capacity building challenges especially when it comes to targeting mid-career investigators.
III organized and participated in multiple workshops and conferences and public outreach has been one of III’s main areas of focus. Moreover, under the leadership of the current SD, III has been excellent in establishing and maintaining partnerships domestically and internationally. The development of communication plan is advisable, however, since the Institute’s profile and activities seem to be well known mainly among previous grant recipients and the regular workshops and conferences participants.
Despite budgetary constraint and changes within CIHR, III’s pursuit of its strategic and operational plans has led to progress and the implementation of initiatives in various areas.
V. References
- Government of Canada, Budget 2018–Equality + Growth: a Strong Middle Class, 2018
- Investing in Canada’s Future: Strengthening the Foundations of Canadian Research. Canada’s Fundamental Science Review (2017)
- CIHR, III, Strategic Plan 2013-2018
- The Government of Canada invests over $21M in innovative health research
- Evaluation of the CIHR Clinical Trials Network in HIV/AIDS Program
- Health Research Rapid Response: Ebola
- CIHR, III, III Newsletter Volume 16, Number 2, June 2017
- Overview of the Reforms to CIHR’s Open Suite of Programs
VI. Appendices
Appendix 1: III Review Panel Members’ Affiliations and Conflict of Interest Declaration
Chair:
- B. Brett Finlay, Professor, Microbiology and Biochemistry, Michael Smith Laboratories, University of British Columbia
Panel Members:
- Megan Levings, Professor, Department of Surgery, University of British Columbia
- Allison McGeer, Professor, Faculty of Laboratory Medicine & Pathobiology; Dalla Lana School of Public Health, University of Toronto
- Claude Perreault, Professor, Department of Medicine, University of Montreal
| Panel Member | Conflict of Interest Declaration |
|---|---|
| B. Brett Finlay | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
| Megan Levings | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
| Allison McGeer | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
| Claude Perreault | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
Appendix 2: Overview of Data Sources and Methods
| Data source | Description |
|---|---|
| Situational Analysis (SA) |
|
| Key informant interviews |
|
| Bibliometric Analysis |
|
Appendix 3: Key Figures and Tables
- Figure A: CIHR Investment in III Mandate by 2012-13 to 2016-17 Research Priorities
- Figure B: Investment in Capacity Building out of III Budget
- Figure C: Percentage of Direct Trainees Funded under III Mandate
- Figure D: Percentage of Indirect Trainees Supported under III Mandate
- Figure E: Leverage Ratio of Partnership to CIHR Investment in III Mandate
- Figure F: Utilization of Institute Support Grant (ISG) Budget
Figure A: CIHR Investment in III Mandate by 2012-13 to 2016-17 Research Priorities
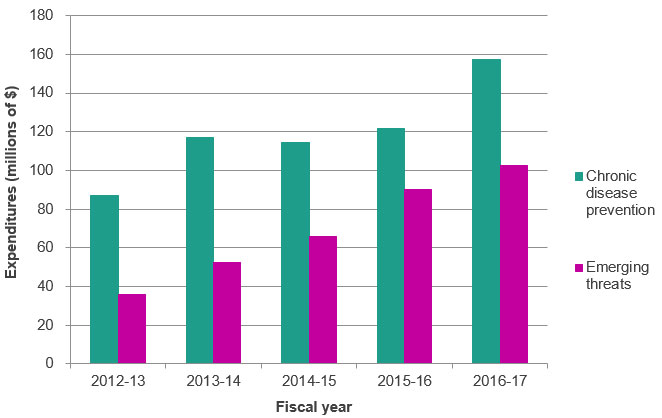
Figure A – Long description
| 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | 2016‑17 | |
|---|---|---|---|---|---|
| Chronic disease prevention | $87,201,569 | $117,231,209 | $114,352,642 | $121,535,693 | $157,219,735 |
| Emerging threats | $35,859,451 | $52,366,918 | $65,698,995 | $90,122,647 | $102,475,196 |
- From 2012-13 to 2016-17, CIHR mainly invested in the Chronic Disease Prevention research priority. CIHR investment in Chronic Disease Prevention increased from $87 M in 2011-12 to $157 M in 2016-17.
- From 2012-13 to 2016-17 CIHR also invested in the Emerging Threats research priority. CIHR investment in Emerging Threats increased from $36 M in 2012-13 to $102 M in 2016-17.
Figure B: Investment in Capacity Building out of III Budget
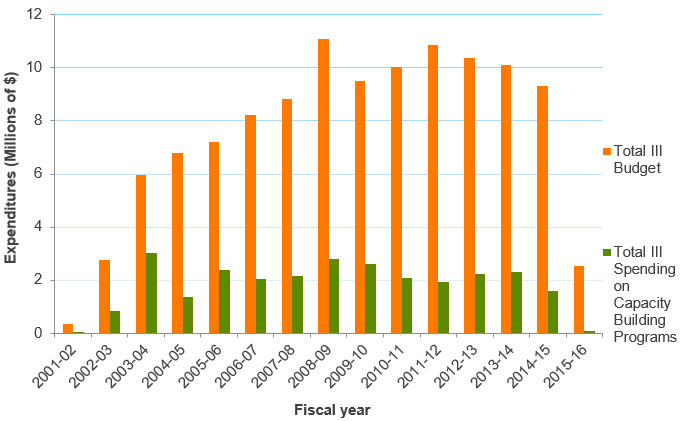
Figure B – Long description
| 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total III Spending on Capacity Building Programs | $58,876 | $820,372 | $3,019,579 | $1,354,537 | $2,374,232 | $2,035,722 | $2,140,557 | $2,808,605 | $2,601,572 | $2,072,565 | $1,913,140 | $2,225,810 | $2,320,066 | $1,600,932 | $73,785 |
| Total III Budget | $335,813 | $2,747,190 | $5,976,006 | $6,801,645 | $7,214,511 | $8,233,046 | $8,815,396 | $11,105,275 | $9,520,751 | $10,031,194 | $10,846,126 | $10,383,021 | $10,093,588 | $9,301,903 | $2,525,111 |
- Between 2001-02 and 2015-16, III’s investments in capacity building activities averaged $1.8 M per year. The figure reached a peak of $3 M in 2003-04.
- III’s spending on capacity building programs out of its budget from 2010-11 to 2014-15 was between $2.1 M (19%) and 1.6 M (6%). In 2015-16 the III spending on capacity building fell to $75 k (3%) as the budget itself decreased to $4.3 M due to the creation of the RAF.
Figure C: Percentage of Direct Trainees Funded under III Mandate
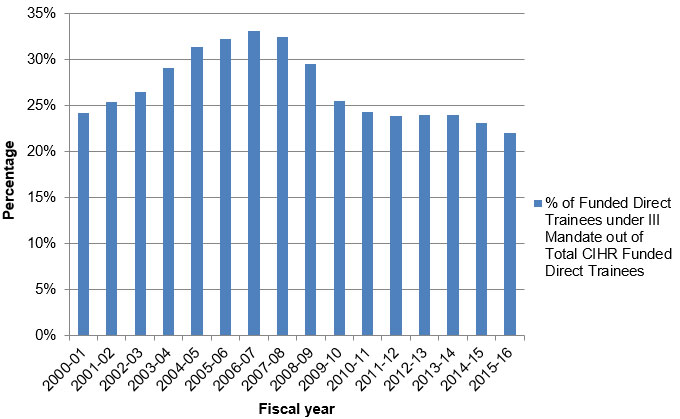
Figure C – Long description
| 2000‑01 | 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Funded Direct Trainees under III Mandate out of Total CIHR Funded Direct Trainees | 24% | 25% | 27% | 29% | 31% | 32% | 33% | 32% | 30% | 26% | 24% | 24% | 24% | 24% | 23% | 22% |
- The annual number of Direct Trainees funded under III’s mandate ranged from 376 in 2000-01 to 764 in 2009-10.
- The number of Direct Trainees funded under III’s mandate, as a proportion of the total CIHR-funded direct trainees, ranged from 24% to 33% between 2000-01 and 2006-07. The maximum (33%) was achieved in 2006-07 with a steady decline since 2006-07 to reach the minimum share (22%) in 2015-2016.
Figure D: Percentage of Indirect Trainees Supported under III Mandate
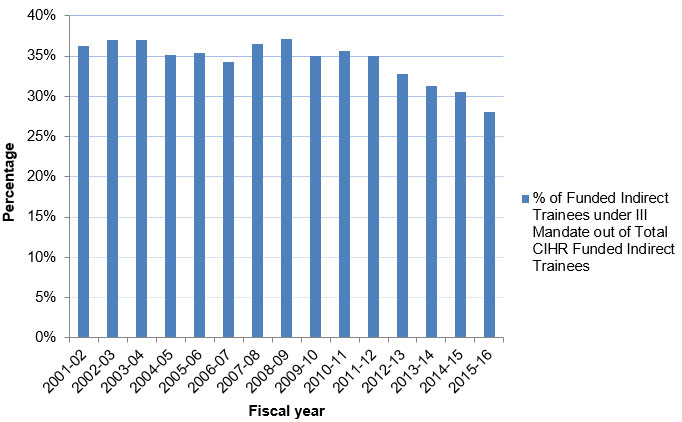
Figure D – Long description
| 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Funded indirect Trainees under III Mandate out of Total CIHR Funded Indirect Trainees | 35% | 36% | 36% | 34% | 35% | 33% | 35% | 36% | 34% | 35% | 34% | 32% | 30% | 30% | 27% |
- The number of Indirect Trainees funded under III’s mandate increased from 2001-02’s 886 to 2,327 in 2009-10. The figure fell from 2,319 in 2011-12 to 1,554 in 2015-16. A decrease is also observed for CIHR funded trainees in the later years.
- The average annual percentage of the Indirect Trainees funded under III’s mandate out of the total number of those funded by CIHR was 34% over the past 15 years.
Figure E: Leverage Ratio of Partnership to CIHR Investment in III Mandate
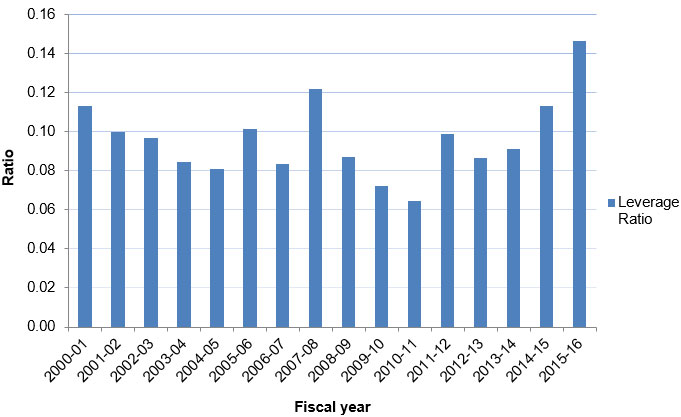
Figure E – Long description
| 2000‑01 | 2001‑02 | 2002‑03 | 2003‑04 | 2004‑05 | 2005‑06 | 2006‑07 | 2007‑08 | 2008‑09 | 2009‑10 | 2010‑11 | 2011‑12 | 2012‑13 | 2013‑14 | 2014‑15 | 2015‑16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leverage Ratio | 0.11 | 0.10 | 0.10 | 0.08 | 0.08 | 0.10 | 0.08 | 0.12 | 0.09 | 0.07 | 0.06 | 0.10 | 0.09 | 0.09 | 0.11 | 0.15 |
- The leverage ratio of partnership to CIHR investment shows how much was invested in III’s mandate through partner contributions for every dollar of CIHR investment in III’s mandate areas.
- In III’s first three years of operation, the annual leverage ratio ranged between 0.10 and 0.11. From 2003-04 to 2006-07, the ratio hovered between 0.08 and 0.10, whereas in 2007-08, the figure reached 0.12. In 2015-16, the ratio reached a 16 year peak of 0.15.
- For the entire 16 year period, III’s average annual leverage ratio was 0.10.
Figure F: Utilization of Institute Support Grant (ISG) Budget
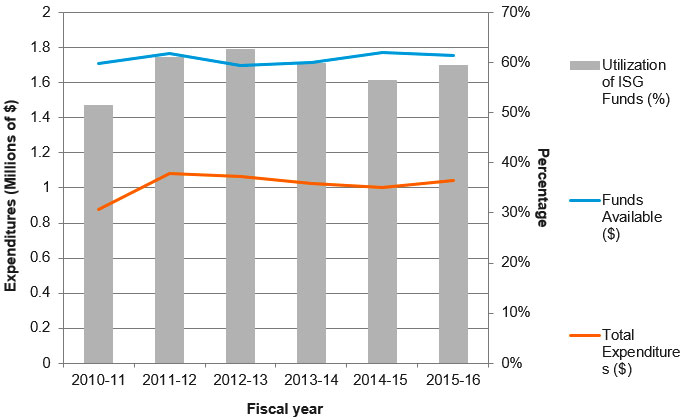
Figure F – Long description
| Year | Funds Available ($) | Total Expenditures ($) | Utilization of ISG Funds (%) |
|---|---|---|---|
| 2010‑11 | $1,710,253 | $878,535 | 51% |
| 2011‑12 | $1,769,963 | $1,080,973 | 61% |
| 2012‑13 | $1,698,087 | $1,065,491 | 63% |
| 2013‑14 | $1,716,686 | $1,026,029 | 60% |
| 2014‑15 | $1,772,143 | $1,001,046 | 56% |
| 2015‑16 | $1,754,534 | $1,042,854 | 59% |
- CIHR annually provides $1 M in Institute Support Grants (ISGs) to each of its 13 institutes in order to establish and sustain their operations, events and collaborative activities. The ISG funds are managed by the host institution which, in the case of III, is Université Laval.
- Between 2010-11 to 2015-16, the main expenditures were as follows:
- III spent an annual average of 74% on Institute Operations.
- An annual average of 26% was spent on Institute Strategic Development (ISD).
- The ISD spending breakdown is as follows:
- Spending on conferences, symposia and workshops averaged 39% of ISD expenditures.
- Other travel, accommodation & hospitality averaged 31% of ISD expenditures.
- Expenditures made in order to operate the Institute Advisory Board (IAB) averaged 20% of ISD expenses.
- Historically, III has spent between 51% and 61% of the annual funds available under the ISG. The smallest (51%) and greatest (63%) proportions were registered in 2010-11 and 2012-13, respectively.
Appendix 4: Sample list of Partners
- Global Affairs Canada (GAC);
- Public Health Agency of Canada (PHAC);
- Correctional Services Canada;
- Natural Sciences and Engineering Research Council (NSERC);
- International Development Research Centre (IDRC);
- Health Canada;
- CIHR Institutes;
- Mount Sinai Hospital’s Lunenfeld-Tanenbaum Research Institute;
- University of Toronto;
- Canadian Blood Services;
- Canadian Liver Foundation;
- Cystic Fibrosis Canada;
- Genome Canada;
- Genome BC;
- Genome Québec;
- Ontario Genomics
- Fonds de recherche du Québec – Santé;
- Kidney Foundation of Canada;
- Canadian Foundation for AIDS Research (CANFAR);
- Canadian HIV Cure Enterprise (CanCURE);
- Bill and Melinda Gates Foundation (BMGF);
- International AIDS Vaccine Initiative Inc.;
- Ministry of Science and Technology of the People’s Republic of China;
- UK Medical Research Council
- International AIDS Society;
- World Health Organizations (WHO);
- Israel Science Foundation;
- Azrielli Foundation;
- Saudi Arabia;
- Coalition for Epidemic Preparedness Innovations;
- UK Health Protection Agency (now a part of Public Health England);
- Canadian Association for HIV Research (CAHR);
- Canada Excellence Research Chair on microbiome;
- Calcul Québec;
- Global Research Collaboration for Infection Disease Preparedness (GloPID-R);
- Joint Programming Initiative on Antibiotic Resistance (JPIAMR);
- National Research Council (NRC);
- European Commission;
- Canadian Food Inspection Agency;
- Allergy, Genes and Environment Network;
- Innovative Medicines Canada; and,
- Alberta Innovates.
- Date modified: