Evaluation of the HIV/AIDS and STBBI Research Initiative (RI)
Final Evaluation Report
May 2025
At the Canadian Institutes of Health Research (CIHR), we know that research has the power to change lives. As Canada's health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
Canadian Institutes of Health Research
160 Elgin Street, 9th Floor
Address Locator 4809A
Ottawa, Ontario K1A 0W9
This publication was produced by the Canadian Institutes of Health Research. The views expressed herein do not necessarily reflect those of the Canadian Institutes of Health Research.
Acknowledgements
Special thanks to all participants in this evaluation – survey respondents, and key informant interview participants. Also, thank you to those who supported the evaluation: Danica Dahlin and Sarah Boorman (Ference and Co.), staff from the Initiative Management and Institute Support (IMIS), Funding Analytics, Financial Planning and Advisory Services and members of the HIV/AIDS and STBBI Research Initiative Working Group and Evaluation Advisory Committee as well as members of the Institute Team and the Scientific Director from the Institute of Infection and Immunity (III), Dr. Charu Kaushic.
The RI Evaluation Team
Gedeon Djissa, Angel Mackenzie, Alice Ndayishimiye, Alison Croke, Jean-Christian Maillet and Michael Goodyer
For more information and to obtain copies, please contact: evaluation@cihr-irsc.gc.ca.
Table of Contents
- List of Tables
- List of Figures
- List of Acronyms
- Executive Summary
- Program Profile
- About the Evaluation
- Evaluation Findings
- Conclusions and Recommendations
- Appendix A: Tables
- Appendix B: Figures
- Appendix C: HIV/AIDS & STBBI Research Impact Summaries
- References
- Endnotes
List of Tables
List of Figures
- Figure 1: CIHR Investment in HIV/AIDS and STBBI
- Figure 2: CIHR RI Investment by Funding Stream
- Figure 3: Top 10 countries on HIV/AIDS and STBBI research Specialization Index (2004-2022)
- Figure 4: Top 10 countries by the Average of Relative Citations (ARC) of Publications in All STBBI areas (2004-2022)
- Figure 5: Number of RI publications by citation in policy
- Figure 6: Number of publications by citations in patents
List of Acronyms
| Acronym | Meaning |
|---|---|
| AIDS | Acquired Immunodeficiency Syndrome |
| CAHR | Canadian Association for HIV Research |
| CanCURE | Canadian HIV Cure Enterprise |
| CanHepC | Canadian Network on Hepatitis C Research |
| CBR | Community-Based Research |
| CIHR | Canadian Institutes of Health Research |
| CTN | CIHR Canadian HIV Trials Network |
| CTRN | CIHR Canadian HIV Trials Research Network |
| EDI | Equity, Diversity and Inclusion |
| FI | Federal Initiative to address HIV/AIDS in Canada |
| FTE | Full-Time Equivalent |
| GBA+ | Gender-based Analysis Plus |
| gbMSM | Gay, Bisexual, and other Men who have Sex with Men |
| HIV | Human Immunodeficiency Virus |
| HSPH | Health Services and Population Health |
| III | Institute of Infection and Immunity |
| IMIS | Initiative Management and Institute Support Branch |
| INHSU | International Network on Health and Hepatitis in Substance Users |
| PHAC | Public Health Agency of Canada |
| PMEC | Performance Measurement Evaluation Committee |
| PWLE | People with Lived Experience |
| RI | HIV/AIDS and STBBI Research Initiative |
| STBBI | Sexually Transmitted and Blood-Borne Infections |
| TBS | Treasury Board of Canada Secretariat |
| WHO | World Health Organization |
Executive Summary
Program Overview
The CIHR HIV/AIDS and STBBI Research Initiative (RI) was launched in 2005 and was initiated as part of the major Government of Canada's response to HIV/AIDS. In 2018, the RI expanded its scope to include other STBBI to align with the Government of Canada's Pan-Canadian STBBI Framework for Action, and Five-year action plan on STBBI. The RI is scientifically led by the Institute of Infection and Immunity (III), and guided by the CIHR HIV/AIDS and STBBI Research Advisory Committee (CHASRAC). The RI provides $21 million of ring-fenced investment annually to support research through four funding streams: Biomedical and Clinical, Health Services and Population Health, Community Based Research (CBR) and Canadian HIV Trials Research Network (CTRN).
Evaluation Purpose, Scope and Methodology
The purpose of this evaluation is to provide CIHR senior management with independent, objective, and actionable findings regarding the relevance, design and delivery, and performance of the RI.
The evaluation covers the period from 2018-19 to 2023-24. It focused on the activities and investments of the RI, including an assessment of the CIHR Hepatitis C Research Initiative, given its alignment with the RI's objectives. The evaluation meets the requirements of the Policy on Results, subsection 42.1 of the Financial Administration Act and the evaluation requirements outlined in the program's authorities.
Key Findings
Relevance
The RI is addressing the need to support HIV/AIDS and STBBI research in Canada. There is a clear, ongoing need to support HIV/AIDS and STBBI research as both HIV/AIDS and STBBI continue to pose a major health challenge in Canada. Notably, infections persist, key populations are disproportionately impacted, and there are emerging, unmet needs (e.g., comorbidities with HIV/AIDS and STBBI).
The RI aligns with federal government responsibilities outlined in the Pan-Canadian STBBI Framework, which emphasizes the role of research and innovation in Canada's response to HIV/AIDS and STBBI. It also supports the CIHR Act by promoting integrated health research, informing decision-making, addressing emerging health challenges, accelerating the discovery of cures and treatments, and advancing the application of research to improve the health of Canadians.
The RI is aligned with the priorities of the federal government as outlined in the Government of Canada's Five-Year Action Plan on STBBI. The RI is also aligned with all five priorities of CIHR's Strategic Plan, and all four strategic goals of the RI Strategic Plan (2022-2027).
Design and Delivery
The evaluation found that the design features support the initiative's objectives by enabling the RI to invest in and adapt to the evolving needs of the multidisciplinary HIV/AIDS and STBBI research landscape. However, the CBR funding stream continues to face challenges in building research capacity within key communities disproportionately affected by HIV/AIDS and STBBI. These challenges could be addressed by expanding eligibility for applicants and community organizations, increasing the use of targeted funding streams, and/or adopting equalization strategies to support researchers from these communities. Through CHASRAC and its subcommittees, the RI has established an effective governance structure to guide the initiative and its Strategic Plan, in line with program authorities.
Available data indicates that the RI is being delivered efficiently, with estimated operating expenditures less than TBS allocations; however, partnerships and community engagement faced challenges due to limited operational resources. Improvements to the monitoring of operational expenditures are needed as CIHR lacks a robust method for tracking FTE associated with RI activities. Performance data is underutilized and contains gaps hindering annual monitoring of initiatives and the communication of impacts. There is also limited evidence that performance reporting is informing program decision-making.
Performance
The RI funds research in priority areas across all four funding streams, and as of 2018-19, it has funded research focused on both HIV/AIDS and other STBBI by expanding the scope of existing funded initiatives (e.g., CTN) and targeted funding opportunities. The RI is building capacity to conduct HIV/AIDS and STBBI research by providing trainees with professional development and networking opportunities that contribute to successful grant applications and career advancement; however, challenges remain in supporting early career researchers.
The RI has advanced knowledge that contributes to the prevention and control of new infections, enhanced the awareness of research findings, and fostered the ability of researchers to engage with community members and knowledge users in all phases of a research project. There are opportunities for CIHR to strengthen engagement with organizations such as PHAC, SSHRC and CATIE would allow to better coordinate knowledge mobilization efforts and support co-funded initiatives aligned with the RI's strategic plan.
The RI-funded research has informed policy and guidelines, improved clinical practice and increased service uptake with notable examples in the advancement of self-testing, informing treatment guidelines and hepatitis C elimination strategies at the provincial level.
There is emerging evidence that RI-funded research is contributing to the prevention of infection and transmission of HIV and STBBI, enhancing the quality of life for individuals living with HIV/AIDS and STBBI as well as contributing to global efforts to reduce the spread of HIV/AIDS and STBBI. Although progress is being made toward achieving these long-term outcomes, more time is needed to fully achieve and assess the impact of funded research.
The COVID-19 pandemic negatively impacted the RI's delivery and performance, delaying key program documents (e.g., the Strategic Plan) and disrupting research activities such as data collection and networking. In response, the RI provided financial support and accommodations to mitigate these challenges.
Recommendations
The evaluation makes four recommendations aimed at improving the performance of the HIV/AIDS and STBBI Research Initiative (RI) to achieve its expected results.
- CIHR should continue to lead the Government of Canada's research response to HIV/AIDS and STBBI to address ongoing and emerging research needs.
- CIHR should update the RI funding mechanisms to minimize application barriers and enhance the capacity of early career researchers and populations disproportionately impacted by HIV/AIDS and STBBI to access RI funding.
- CIHR should strengthen engagement with government and non-governmental organizations to:
- Advance research and build capacity for priority populations disproportionately impacted by HIV/AIDS and STBBI.
- Coordinate knowledge mobilization of RI-funded research to maximize its use by healthcare and community organizations.
- CIHR should improve the availability and monitoring of:
- Operating and maintenance expenditure data, specifically direct salary costs.
- Performance reporting of RI-funded research to better inform decision-making and communicate impact.
Program Profile
The CIHR HIV/AIDS and STBBI Research Initiative (RI)
CIHR's HIV/AIDS and STBBI Research Initiative (RI) was initiated as part of the major Government of Canada's response to HIV/AIDS launched in 2005 through the Federal Initiative to address HIV/AIDS in Canada (FI). The FI was coordinated between four federal departments (i.e., Public Health Agency of Canada [PHAC], CIHR, Health Canada, and Correctional Services Canada), with CIHR managing the research components of the FI. The RI provides $21 million of ring-fenced investment annually RI funding is disbursed through four funding streams: Biomedical and Clinical Research, Health Services and Population Health Research, Community-Based Research, CIHR Canadian HIV Trials Research Network.
In 2018, other sexually transmitted and blood borne infections (STBBI) were added to the scope of the RI, in alignment with the Government of Canada's integrated approach to HIV and STBBI across the full continuum of prevention, testing, initiation of care and treatment and ongoing care. Indeed, HIV/AIDS and STBBI research funded by CIHR is intended to align with the priorities described in the federal government's Pan-Canadian STBBI Framework for Action (2018), and the Government of Canada five-year action plan on STBBI (2019). The Pan-Canadian STBBI Framework for Action provides a roadmap for collaborative and complementary actions to reduce the impact of STBBI in Canada and to contribute to the global efforts to end AIDS, viral hepatitis, and sexually transmitted infections as major public health concerns.
The RI's mission outlined in its Strategic Plan 2022-2027 is to strengthen and support a diverse, inclusive and collaborative research community that applies community-based, holistic and inter- and transdisciplinary approaches to create and mobilize knowledge for better and equitable prevention, testing, treatment and care of HIV/AIDS and STBBI in Canada and around the world. The RI is scientifically led by the Institute of Infection and Immunity (III), and guided by the CIHR HIV/AIDS and STBBI Research Advisory Committee (CHASRAC), which is comprised of members representing various areas of HIV/AIDS and STBBI research, government, and HIV/AIDS and/or STBBI community organizations.
Hepatitis C Research Initiative
The Hepatitis C Research initiative is a partnership between CIHR and the Public Health Agency of Canada (PHAC). Under this partnership, PHAC transfers $900,000 annually to CIHR to administer and support the investment in this initiative. The Canadian Network on Hepatitis C Research (CanHepC) was the successful project during the evaluation period. Its objective is to create a cohesive, collaborative research program in Canada linking researchers, knowledge users and decision makers from multiple pillars and jurisdictions across the country by conducting biomedical and clinical research as well as research with direct public health relevance.
About the Evaluation
Purpose and Scope
The purpose of this evaluation was to provide CIHR senior management with independent, objective, and actionable findings regarding the RI to inform planning and decision-making. The evaluation meet the evaluation requirements of the Policy on Results, subsection 42.1 of the Financial Administration Act, and the program's authorities. The evaluation focused on assessing the following aspects of the RI:
- Relevance: The needs addressed by the RI, and its alignment with roles and responsibilities of federal government as well as with CIHR's mandate and strategic priorities.
- Design and Delivery: The effectiveness of the implementation, design, and governance structure in achieving program objectives; and
- Performance: The extent to which the program has achieved its expected outputs, as well as its immediate, intermediate, and ultimate outcomes.
The evaluation focused on the activities and investments of the HIV/AIDS and STBBI RI between 2018-19 and 2023-24, though it should be noted that, at the time of data collection for the evaluation, administrative data were only available up to the 2022-23 fiscal year. During this period, the RI funded 343 grants and awards, representing a total investment of $107 million. The evaluation will focus on 191 grants ($106 million) funded across the RI's four funding streams (Biomedical and Clinical Research, Health Services and Population Health Research, Community-Based Research, and the CIHR Canadian HIV Research Trials Network). The remaining 152 grants ($1 million), which include primarily planning and dissemination grants and travel awards, were not in scope for this evaluation given the overall low materiality of these investments. The evaluation also assessed the performance of the Hepatitis C Research Initiative, specifically the CanHepC Network, given that its objectives align with those of the RI. The approach to assess the RI's performance relied both on the RI's logic model, which was updated in 2015, as well as the RI's current strategic plan, which covers the period from 2022 to 2027. As such, the assessment of the RI's outputs and immediate outcomes were guided by the priorities and goals from the RI Strategic Plan. The assessment of the medium- to long-term outcomes were guided by the corresponding outcomes in the RI's logic model, given that many of these activities would have commenced before the release of the new strategic plan.
Evaluation Questions
The evaluation questions were developed in consultation with and approved by CIHR's Performance Measurement and Evaluation Committee (PMEC) at its June 2023 meeting. The evaluation addresses the following specific questions:
Relevance
- To what extent does RI continue to address an ongoing need?
- To what extent is RI aligned with the federal government and CIHR roles and responsibilities?
- To what extent is RI aligned with federal government and CIHR priorities?
Design and Delivery
- How do the design features of the RI facilitate the achievement of intended objectives?
- Has RI implemented an effective governance and oversight?
- To what extent has RI been efficiently and effectively delivered?
Performance
- To what extent has RI achieved its expected outputs?
- To what extent has RI made progress toward the achievement of its immediate outcomes?
- To what extent has RI made progress toward the achievement of its expected intermediate outcomes?
- To what extent has RI made progress toward the achievement of its expected ultimate outcomes?
COVID-19
- To what extent has COVID-19 pandemic affected the design and delivery and performance of the RI?
Methodology
Consistent with TBS guidelines and recognized best practice in evaluation, a range of methods and data sources were used to triangulate findings for the RI Evaluation. These included document review; administrative and financial data review; bibliometric/altmetric analysis; key informant interviews (n=19) with CIHR Management (n=7, including CHASRAC), Researchers (n=5), Knowledge Users (n=4), and Partners (n = 3); surveys of researchers and trainees; and case studies (n = 5). Gender-based analysis plus (GBA+) and equity, diversity and inclusion (EDI) considerations were built into the evaluation framework via specific evaluation indicators.
The survey of researchers targeted researchers who have received funding (i.e., researchers) or applied but were unsuccessful (i.e., unfunded applicants). The survey of trainees only targeted funded trainees. The resulting survey data consisted of 61 out of 121 researchers (50.4%), 44 out of 137 unfunded applicants (32.1%) and 16 out of 30 trainees (53.3%).
Note that the reported denominator will change as it reflects the number of individuals who were posed the question. Given the large number of lines of evidence with varying sample sizes, the following qualifiers have been used to indicate the frequency of responses for some lines of evidence conducted, for consistency (i.e., surveys and key informant interviews). In the case of interviews, it is important to note that these qualifiers have been used to summarize statements about qualitative data; they should not necessarily serve as a measure of the importance of the respective finding:
| None | A few | Some | Many | Most | Almost all | All |
|---|---|---|---|---|---|---|
| (0 or no) | (<20%) | (20-39%) | (40-59%) | (60-79%) | (80-99%) | (100%) |
Limitations of this Evaluation
- With a very complex and continuously changing funding landscape in RI research, it was difficult to attribute outcomes to the RI and specifically CIHR's role in the RI. As a result, conclusions from this evaluation spoke to CIHR's contribution to the outcomes and impacts identified by the various lines of evidence.
- Some secondary data (e.g., end of grant reports, mid-term and annual reports) were not collected for the purposes of this evaluation specifically (e.g., there was incomplete/dated information). To mitigate this limitation, the evaluation used multiple lines of evidence to fill data gaps to inform the findings and conclusions.
- There was limited availability of, and consistency in, the administrative self-identification data, causing challenges in assessing GBA+ and EDI considerations, owing to the existence of data gaps corresponding to the period under review and an update to CIHR's self-ID questions in July 2022. To partially mitigate this limitation, the evaluation included CIHR's up-to-date self-ID questions in its researcher survey and used these data to assess EDI considerations.
- There is the potential for biases with key informant interviews (e.g., interviewer bias, selection bias, self-report bias) which could lead to inaccuracies in the data. This limitation is mitigated by triangulating key informant interviews data with researcher survey data and findings from case studies, document review and altmetric analysis.
- Given the specialized nature of the initiative and its components, there was limited available data on counterfactual groups for comparison. To mitigate this limitation, survey data have been used, to the extent possible, to compare funded researchers with unfunded applicants, who served as a comparable group.
- The analysis of the RI's performance data was limited by the extent of the mid- and end- of grant reporting available for the period under review. For example, as a result of the COVID-19 pandemic, a number of funded grants were not completed and had not yet submitted mid-term or end of grant reports at the time of data collection. To mitigate this limitation, the evaluation used multiple lines of evidence to fill data gaps to inform the findings and conclusions.
Evaluation Findings
Relevance
Key Findings:
- The RI is addressing the need to support HIV/AIDS and STBBI research in Canada. However, there is a clear, ongoing need to support research as HIV/AIDS and STBBI continue to pose major public health challenges in Canada. Notably, infections persist, key populations are disproportionately impacted, and there are emerging, unmet needs (e.g., comorbidity of mental health and HIV and STBBI).
- The RI is aligned with the roles and responsibilities of the federal government, with the Pan-Canadian STBBI Framework identifying responsibilities to facilitate research and innovation as part of the Government of Canada's response to HIV/AIDS and STBBI.
- The RI aligns with the CIHR Act in supporting research that forges an integrated health research approach and informs decision-making, addresses emerging health threats and challenges and accelerating the discoveries of cures and treatments as well as promoting the dissemination of knowledge and the application of health research to improve the health of Canadians.
- The RI is aligned with the priorities of the federal government as outlined in the Government of Canada's Five-Year Action Plan on STBBI. The RI is also aligned with all five priorities of CIHR's Strategic Plan, and all four strategic goals of the RI Strategic Plan (2022-2027).
There is a continued need to support HIV/AIDS and STBBI Research in Canada
There is a clear and ongoing need to support HIV/AIDS and STBBI research in Canada as HIV/AIDS and STBBI continue to pose major public health challenges. In 2023, 39.9 million people were living with HIV/AIDS around the world, with 1.3 million new infections in 2023, 14% of individuals unaware of their HIV positive status, and 630,000 deaths (WHO, 2024). Over 1 million new cases of four curable sexually transmitted infections (i.e., chlamydia, gonorrhea, syphilis and trichomoniasis) occur every day worldwide (WHO, 2022).
In Canada, infection rates are on the rise. In 2023, 2,434 people were newly diagnosed with HIV, a 35% increase since 2022 (PHAC, 2024a). Similarly, rates of other STBBI have been increasing (e.g., Chlamydia: 22% increase between 2012 and 2019; Gonorrhea: 151% between 2012 and 2019; Syphilis: 109% between 2018 and 2022) (PHAC, 2024b). Moreover, specific communities continue to be disproportionally impacted. Indeed, gay, bisexual, and other men who have sex with men (gbMSM) represent 39.7% of new HIV diagnoses, people who inject drugs represent 21.9%, Indigenous Peoples represent 23.9% and Black populations represent 15.4% (PHAC, 2024b)Footnote 1. Up to 85% of new hepatitis C infections in 2019 occurred among people who use drugs (PHAC, 2019), and gbMSM represented 27% of all reported infectious syphilis cases in 2022 (PHAC, 2024b). Stigma and discrimination profoundly impact those marginalized groups, exacerbating their vulnerability to STBBI (PHAC, 2019).
In the face of the increasing burden of HIV/AIDS and STBBI there is a clear need for continued investments in HIV/AIDS and STBBI research. To this end, CIHR has invested $280 million dollars in HIV/AIDS and STBBI research between 2018-19 and 2022-23, with $141.6 directed towards research in priority areas, including the RI, $128.4 million in investigator-initiated research and $10 million in training and career support. In the same period, CIHR invested $107 million via the RI, which, on average, accounts for 38% of CIHR's total investments in HIV/AIDS and STBBI research (Figure 1: CIHR Investment in HIV/AIDS and STBBI). Despite these investments, a couple of key informants (n=2 out of 7) from CIHR Management observed that the expanded scope of the RI to include all STBBI poses a challenge for the RI to meet the increased HIV/AIDS and STBBI research needs with the same level of funding.
CIHR's investments in HIV/AIDS and STBBI research point to Canada's strong and competitive research ecosystem in the field. A bibliometric analysis of Canada's HIV/AIDS and STBBI research found that Canada produced 12,912 publications between 2004 and 2022, ranking 7th in global output, and on average accounts for 5.1% of the global share of publications. Similarly, Canada ranked 3rd globally on the specialization index for HIV/AIDS and STBBI research (Figure 3: Top 10 countries on HIV/AIDS and STBBI research Specialization Index (2004-2022)), and Canadian publications are cited at a rate higher than the world average (ranking 3rd globally, Figure 4: Top 10 countries by the Average of Relative Citations (ARC) of Publications in All STBBI areas (2004-2022)).
All key informants across respondent categories (n=19 out of 19) agreed that the RI is meeting some of the research needs for HIV/AIDS and STBBI in Canada. Notably, some key informants (n=5 out of 19) across respondent categories emphasized community-based research support as a key strength of the RI, noting that it enhances the response to community needs and delivers more direct research impacts. Additionally, researchers interviewed (n=3 out of 5) reported that the RI is filling key research gaps including engagement of people with lived experience (PWLE) in the research process and knowledge dissemination to the broader HIV/AIDS and STBBI community. These findings are supported by the researcher survey that demonstrates that on average half of all researchers (50% of 60, M = 3.52 out of 5, SD = 0.11), and unfunded applicants (52% of 42, M = 3.62 out of 5, SD = 0.11) reported that the RI funding is addressing a need for HIV/AIDS and STBBI research in Canada to a great or very great extent. Further, almost two thirds of recipient researchers (65% out of 57) reported that their projects would not have proceeded if they had not received the RI funding, and nearly two thirds of unfunded applicants (61% out of 41) reported that their proposed project did not proceed in the absence of the RI funding.
There is evidence of some needs not being addressed. Nearly three quarters of both researchers (72% out of 57) and unfunded applicants (71% out of 41) surveyed reported that there are needs not being addressed by the RI. Most researchers surveyed (70% out of 57) cited a variety of unmet needs, including: insufficient or lack of sustained funding, a lack of research focused on rural and remote communities, and the co-morbidity of mental health disorders with HIV/AIDS and STBBI.
In addition to unmet needs, several emerging needs in HIV/AIDS and STBBI research were identified and highlight the importance of ongoing support for this type of research. A review of program documents underscores the need to better understand the intersectionality of COVID-19 and HIV and STBBI (CIHR, 2021c), and address the growing challenge to effectively treat drug-resistant STI such as gonorrhea (PHAC, 2018). The document review findings are supported by interview findings with almost all key informants (n= 18 out of 19) identifying a continued need for research to address HIV/AIDS and STBBI and many interviewees (n=13 out of 19) highlighting emerging areas such as HIV and aging, STBBI infections and mental health as well as other comorbidities, and the disproportionate impact on priority populations, including Indigenous People and racialized communities.
RI is aligned with the roles and responsibilities of the federal government and CIHR.
The RI is aligned with the roles and responsibilities of the federal government in supporting HIV/AIDS and STBBI research in Canada as outlined in the Pan-Canadian STBBI Framework for Action. Released in 2018, the framework defines the federal government response to HIV/AIDS and STBBI and its role in promoting and protecting health, including sexual health, and specifically its responsibilities to facilitate research and innovation to understand STBBI transmission and to support the development of new diagnostic tools (PHAC, 2018).
Further, CIHR's role in supporting HIV/AIDS and STBBI research through the RI is directly aligned with the CIHR Act (S.C. 2000, c6).Specifically, the RI aligns with several of CIHR's objectives as outlined in the Act including: (4c) 'forging an integrated health research agenda across disciplines, sectors and regions that reflects the emerging health needs of Canadians and the evolution of the health system and supports health policy decision-making'; (4f) 'addressing emerging health opportunities, threats and challenges and accelerating the discovery of cures and treatments and improvements to health care, prevention and wellness strategies' and (4h) ‘promoting the dissemination of knowledge and the application of health research to improve the health of Canadians.
Overall, most key informants (n=18 out of 19) stated that there was a clear role for the federal government to support HIV/AIDS and STBBI research. Indeed, partners (n=2 out of 3) reflected that through its role in supporting research the federal government would foster its involvement in the HIV/AIDS and STBBI community, specifically related to treatment and prevention efforts. Further, researchers (n = 4 out of 5) and knowledge users (n = 4 out of 4) highlighted CIHR's critical role in supporting the continued HIV/AIDS and STBBI research needs.
RI is aligned with the federal government and CIHR's strategic priorities.
The RI aligns with the federal government's strategic priorities as outlined in the STBBI Five-Year Action Plan. The action-plan launched in 2019 and updated in 2024, provides a roadmap for the implementation of the federal government's response to HIV/AIDS and STBBI outlined in the Pan-Canadian STBBI Framework for Action. The action plan identifies three strategic goals: 1) to reduce the incidence of STBBI in Canada, 2) improve access to testing, treatment, and ongoing care and support, and 3) reduce stigma and discrimination that create vulnerabilities to STBBI (PHAC, 2019; PHAC 2024b). CIHR is identified as the lead department for research-oriented actions within the Prevention, Testing, and Ongoing Care and Support pillars.
The RI's objectives are also aligned with all five priorities of CIHR's Strategic Plan 2021-2031 aiming to achieve the CIHR's vision of "the best health for all, powered by outstanding research". Specifically, the RI's objectives, in particular those are closely aligned with priority (A) Advance research Excellence in all its Diversity (B) strengthen Canadian health research capacity; (C) accelerate the self-determination of Indigenous Peoples in health research; (D) pursue health equity through research; and (E) integrate evidence in health decisions (CIHR, 2021e).
Further, the RI's activities and investments are guided by the RI's Strategic Plan (2022-2027) and its four strategic goals: Improve Health Equity, Accelerate Discovery and Innovation, Strengthen Research Capacity, and Mobilize Knowledge. A few key informants from CIHR Management (n=3 out of 7) highlighted the close alignment between the RI's strategic plan and CIHR's strategic plan ensuring that key RI knowledge creation and dissemination activities are also aligned with CIHR's priorities. Additionally, these key informants highlighted the RI's strategic plan was used as a key input in the development of the renewal of the STBBI Five-Year Action Plan (2024-2030) thereby ensuring an alignment of the RI's activities with federal government priorities.
Design and Delivery
Key Findings:
- The design features facilitate the achievement of intended objectives allowing the RI to invest across HIV/AIDS and STBBI's multidisciplinary landscape as well as to adapt to the evolving needs of the HIV/AIDS and STBBI research landscape.
- Challenges persist in the CBR funding stream related to building research capacity within key communities disproportionately impacted by HIV/AIDS and STBBI. Addressing these challenges can include expanding eligibility requirements for both applicants and community organizations, increasing the use of specific funding streams and/or adopting equalization approaches to foster capacity building among researchers from communities disproportionately impacted by HIV/AIDS and STBBI.
- Through CHASRAC and its subcommittees, the RI has implemented an effective governance structure to guide the development and implementation of the initiative and its Strategic Plan, consistent with requirements outlined in the program authorities.
- Available data indicates that the RI is being delivered efficiently, with the estimated operating expenditures less than TBS allocated expenditures. CIHR should improve the monitoring of operational expenditures as it currently lacks a robust method for tracking FTE associated with RI activities. There is also limited evidence that performance reporting is informing program decision-making.
RI's funding streams facilitate the achievement of its intended objectives
The RI program authorities outlined that the initiative support research across four different priority streams:
- Biomedical and Clinical: This stream invests in research aimed at developing effective prevention, treatment, and cure strategies to improve the health of Canadians affected by HIV/AIDS and STBBI;
- Health Services and Population Health (HSPH): Supporting researchers and community centres to improve health and access to effective programs and services for people living with and at risk of STBBI, particularly for populations most affected by HIV/AIDS and STBBI;
- Community-Based Research (CBR): Focuses on building partnerships between community leaders and researchers to conduct research and capacity-building initiatives; and
- Canadian Trials Research Network on HIV/AIDS and STBBI (CTRN): Designed to accelerate the translation and uptake of new knowledge into clinical practice and guidelines for HIV/AIDS and other STBBI. It also supports building research infrastructure and offering services that enable HIV/AIDS and STBBI investigators to conduct clinical trials, and that strengthen Canadian research capacity.
A review of funding opportunities across these fundings streams highlights the RI's ability to invest across the HIV/AIDS and STBBI multidisciplinary research landscape while aligning the initiative's objectives with federal government priorities as outlined in the STBBI Action Plan.
These findings are supported by the survey results with three-quarters of both funded researchers (75% out of 56) and unfunded applicants (78% out of 32) agreeing to a great or very great extent that the RI funding facilitated multidisciplinary and/or intersectoral collaborations that would not have occurred otherwise. All Researchers above and CIHR Management (n=12 out of 12) indicated that the funding streams allow the RI to meet diverse research needs in the HIV/AIDS and STBBI community with some respondents (n=5 out of 12) specifically stating that the streams support multidisciplinary research. However, some members of CIHR Management (n=3 out of 7) observed that it is becoming increasingly challenging to support multidisciplinary research and maintain a balance of funding across the funding streams.
Some key informants (n=6 out of 19) across all respondent categories highlighted the ability of the RI funding streams to adapt to changing research needs. This is exemplified by the inclusion of the other STBBI into the scope of the RI in 2018-19, which had been previously predominantly focused on HIV/AIDS (CIHR 2019). Additionally, the RI updated the CTRN funding stream to respond to the evolving changes in the clinical trials landscape and to better align its objectives to the new RI Strategic Plan (2022-2027). Specifically, it expanded the scope of the CTRN to include all STBBI, strengthened inclusion of people with lived experience, implemented a new pan-Canadian governance model and enabled engagement with other CIHR strategic initiatives such as the Clinical Trials Fund (CTF) and the Strategy for Patient Oriented Research (SPOR) (CIHR 2023d).
Overall, while the RI's funding streams support the achievement of its objectives, challenges have been identified in the CBR funding stream related to the development of research capacity particularly within populations disproportionately impacted by HIV/AIDS and STBBI. A review of program documents found low application pressure in the CBR Indigenous Stream with some rounds receiving no applications. An internal review of the CBR general streams by CHASRAC highlighted areas for improvement including a lack of community-led and community-driven research and a lack of representation from minoritized communities. As the RI begins its phased implementation of the CBR refresh, CHASRAC has advised that the initiative explore innovative strategies to remove barriers to access funding and engagement including a continued use of distinctions-based approaches and examining the eligibility of applicants and organizations to receive funds (CIHR 2022a). These findings are supported by the views of some key informants (n=10 out of 19) who highlighted the need to address capacity gaps, specifically within Indigenous and racialized communities, to increase participation in research to better address community needs. Related to this, many other key informants (n=8 out of 19) highlighted the need to provide funding directly to community organizations, particularly within specific communities that are disproportionately impacted by HIV/AIDS and STBBI, to enhance community-led or community-driven research and knowledge mobilization.
CIHR should continue to make use of strategies such as distinctions-based approaches in funding opportunities to help build capacity among priority populations. Further, collaboration across relevant CIHR business units such as Program Design and Delivery, Funding Opportunities Management and Finance is essential to update funding policies, which could include expanding the eligibility requirements for both applicants and community organizations, increasing the use of specific research streams or adopting equalization approaches to foster capacity building among researchers from communities disproportionately impacted by HIV/AIDS and STBBI.
RI has implemented effective governance through CHASRAC.
The RI's program authorities identify CHASRAC as the governing body to guide the initiative. As outlined in the committee's terms of reference, its roles and responsibilities include providing guidance, make recommendations regarding research priorities and strategic research, and ensure the inclusion of priority populations in decision making (CIHR, 2021d). CHASRAC provides guidance on the implementation of the RI and advises the Scientific Director of III and CIHR's Vice-President, Research Programs on funding opportunity development and the implementation of the RI's Strategic Plan (CIHR, 2021d). The committee also regularly consults the HIV/AIDS and STBBI community to inform the strategic directions of the research initiative.
Moreover, CHASRAC has established subcommittees for the CTRN and CBR programs to provide focused advice on these funding streams and advance their objectives. Program documents highlighted the important contribution of the CTRN sub-committee in addressing the evolving needs of the Canadian clinical trials landscape, including new challenges, considerations, and emerging opportunities (CIHR, 2023a). Similarly, the CBR subcommittee has examined the program's low application pressure and proposed changes reflected in the RI's implementation phased approach (CIHR, 2021b).
These findings suggest that CHASRAC is effectively guiding the RI, which is corroborated by many (n=6 out of 12) key informants from CIHR Management and researcher respondent groups who noted that CHASRAC and its governance structure were contributing effectively to decision-making for the RI, by engaging relevant communities, consulting CIHR Equity Strategy Team and coordinating with III. Some members of CIHR Management (n=3 out of 7) highlighted CHASRAC as an important governance mechanism for the RI, emphasizing its commitment to engaging community members.
RI is being delivered efficiently, however, there are challenges impacting delivery.
Available data indicates that the RI is being delivered efficiently with estimated administrative costs consistently lower than the allocated costs and CIHR's overall administrative costs. Between 2018-19 and 2022-23, the estimated total cost of administering the RI, as a percentage of total program expenditures, varied between 4.0% and 4.3%, which was lower than CIHR's overall of 5% and the RI administrative cost percentage of 6.7% based on allocations in the program authorities. (Table 1: CIHR Allocated (based on TB submissions) and Actual Operating Costs on RI, 2018-19 to 2022-23). The financial data review found that the RI expended, on average, 59% of its operating budget, which includes salary and non-salary expenditures, allocated in the program authorities. The key driver of administrative cost are direct salary costs for FTEs. The estimated total number of FTEs was consistently lower (ranging between 5.7 to 6.3) than the allocated 10 FTEs outlined in the RI program authorities. There is a need to improve the monitoring of administrative expenditures as the financial review found that CIHR lacks a robust system for tracking and estimating FTEs dedicated to RI activities.
Despite the available data indicating that the RI is being delivered efficiently, key informants observed (5 out of 7 members of CIHR Management) that the implementation of key activities, such as the establishment of partnerships and community engagement, which often require an in-person presence, were challenging due to operational resource constraints.
There is limited evidence from the document review that progress and annual reports are informing program decision-making. Moreover, the evaluation found variations in performance reporting across funding opportunities, with some focused predominantly on outputs whereas others included some details on achievements. This is consistent with interview findings with some key informants (n=3 out of 10) representing CIHR Management and partners reporting that performance data is underutilized and has gaps that do not allow for the annual monitoring of initiatives and the communication of impacts. Many members of CIHR Management (n=4 out of 7) highlighted that the annual reports are being used to track investments, however, resource constraints limit the tracking of performance data.
Performance
Key Findings:
- The RI has achieved its expected output by funding research in priority areas across all four funding streams and integrating a research focus on other STBBI by expanding the scope of existing funded initiatives (e.g., CTN) and through targeted funding opportunities.
- The RI is building capacity to conduct research in HIV/AIDS and STBBI that provide trainees with professional development and networking opportunities that contribute to successful grant applications and career advancement, however key challenges in supporting early-career researchers remain.
- The RI is achieving its immediate outcomes by advancing knowledge that contributes to prevention and control of new infections, enhancing the awareness of research findings and fosters the ability of researchers to engage with community members and knowledge users in all phases of the research project. CIHR should strengthen engagement with organizations to coordinate and enhance capacity building, knowledge mobilization and co-fund research.
- The RI is achieving its intermediate outcomes by supporting research that has informed policy and guidelines, improved clinical practice and increased service uptake with notable examples in the advancement of self-testing, informing treatment guidelines and hepatitis C elimination strategies at the provincial level.
- The RI is making progress towards achieving long-term outcomes. There is emerging evidence that the RI is contributing to the prevention of infection and transmission of HIV and STBBI, enhancing the quality of life for individuals living with HIV/AIDS and STBBI, and supporting global efforts to reduce the spread of HIV/AIDS and STBBI.
- The COVID-19 pandemic negatively affected the delivery and performance of the RI including the development of key program documents (e.g., RI's Strategic Plan) and the interruption of research activities (e.g., data collection and networking). Overall, these impacts were mitigated by RI program management.
RI is funding research in priority areas across all four funding streams while integrating a focus on other STBBI.
Between 2018–19 and 2022–23, the RI invested $106.1 million toward priority research areas through four main funding streams. The largest share of funding was allocated to the Biomedical and Clinical stream ($35 million; 29 applications), followed closely by the Health Services and Population Health (HSPH) stream ($32.2 million; 94 applications). The Clinical Trials Research Network received $23.3 million through just 2 applications, while the Community-Based Research (CBR) stream received $15.6 million across 66 applications (Figure 2: CIHR RI Investment by Funding Stream). In addition, CIHR partnered with PHAC to invest $4.5 million during the same period in the Canadian Hepatitis C Network, supporting research focused specifically on Hepatitis C.
Since the scope of the RI expanded to include other STBBI in 2018-19, the RI has been funding research focused on other STBBI. Between 2018-19 and 2022-23, projects with a primary focus on HIV/AIDS research, on average, account for 91% of the RI's total investment, followed by hepatitis C, which accounts for 37.5% and other STBBI (i.e., HPV, Syphilis, Chlamydia and Gonorrhea)Footnote 2 accounting for 1%Footnote 3. It is important to note that according to the administrative data, the RI only began tracking specific investments in other STBBI in 2021-22. Although the review of administrative data indicates that the RI's research investment is still primarily focused on HIV/AIDS, CIHR management (n=2 out of 7) indicated that the RI is increasing efforts to support research focused on other STBBI through existing and new investments (e.g., CTRN, STBBI Research in Canada: Beyond HIV/AIDS and Hepatitis C), while also highlighting that the inclusion of other STBBI is stretching already limited resources.
The RI is supporting diversity among HIV/AIDS and STBBI researchers.
The RI is committed to supporting diversity in HIV/AIDS and STBBI research. Program documents indicate that the RI has prioritized investments to support the research led by First Nations, Inuit and Métis researchers, by allocating $12 million (out of $107 million) within the Health Services and Population Health (HSPH) ($5.9 million) and CBR ($6.1 million) funding streams. Additionally, a review of funding opportunities demonstrates the RI's commitment to the use of a distinctions-based approach, Gender Based Analysis Plus (GBA+) and the Tri-Agency EDI action plan in supporting equitable access to funding opportunities.
The evaluation encountered challenges in assessing the diversity of RI-funded researchers due to gaps in CIHR self-identification data collected by the program during the period under review by the evaluation. To mitigate this limitation, surveyed RI researchers were given the option to provide their demographic information. Of the researchers surveyed 39% (out of 61) identified as men and 46% (out of 61) as women, 21% (out of 61) identified as a visible minority, of which 6.5% identified as being a member of the African, Caribbean and Black Community. Finally, 5% (out of 61) identified as Indigenous. Based on survey responses, noting item non-response, it appears that the RI is making gains in supporting diverse researchers.
However, despite efforts by the RI to support diversity amongst researchers, many key informant (n=8 out of 19) acknowledge that further support is needed for researchers from underrepresented groups disproportionately affected by HIV/AIDS and STBBI to address these capacity gaps. A few respondents (n=2 out of 19) highlighted specific solutions to address these capacity gaps such as addressing barriers faced by Indigenous researchers within academia (e.g., Western research methods challenging Indigenous research methodologies) as well as providing dedicated funding to address other communities disproportionately impacted by HIV/AIDS and STBBI.
RI is building research capacity for HIV/AIDS and STBBI research.
"The people that keep on getting funded are the senior researchers who have been there all this time…so it's about really making sure that you have mechanisms for ensuring that you don't keep on funding senior researchers because they produce the most excellent projects, and the upcoming are always left out."
The RI is building research capacity through both direct training support (e.g., training awards) and indirect support provided by funded researchers. Between 2018-19 to 2022-23, the RI supported 30 direct training awards (e.g., doctoral research and fellowship awards) for a total of $2.1 million. A survey of trainees indicated that nearly all respondents (94% out of 16) reported being employed after their training, and of these, nearly three quarters (73% out of 15) were working in the university sector, with 91% (out of 11) continuing research in HIV/AIDS and STBBI. Additionally, almost all researchers surveyed (85% out of 61) reported that they provided support to trainees as part of their research project, including doctoral students (75% out of 52 researchers), postdoctoral fellows (64% out of 52), Master's students (65% out of 52), and undergraduate students (48% out of 52). These researchers reported that research and technical skill development (M = 4.75 out of 5, SD = 0.48), and professional skill development (94% out of 52, M = 4.58 out of 5, SD = 0.61) were activities that were offered to a great or very great extent. These findings are supported by many key informants (n = 13 out of 19) across respondent groups who reported that the RI played a crucial role in building capacity for trainees, contributing to successful grant applications and career advancement. However, some key informants (n=4 out of 19) across CIHR management, partner and researcher categories observed that there should be more support for early career researchers (ECRs), pointing to challenges in obtaining sufficient funding to establish a program of research and the use of specific funding streams as a mean to address this key gap. It should be noted that CIHR does not track the career stage of applicants, except for the Project Grant Program, thus the evaluation was not able to determine the proportion of RI researchers that are ECRs.
Case studies highlighted several examples of how RI-funded research is supporting capacity building. Notably, the Student Mentorship program of the Aboriginal HIV and AIDS Community-Based Research Collaborative Centre (AHA Centre) mentored graduate students in HIV research within the Indigenous context. This program provided support with grant writing, assisted over 45 grant applications and shared expertise in Indigenous Knowledges and Decolonizing Research methodologies. Other initiatives such as the REACH Universities Without Walls (UWW) provided trainees with hands-on, community-based training opportunities, including field placements at community-based locations to build community-based research capacity.
CanHepC is building trainees' capacity through its Training, Education, and Mentorship Program, which provides multidisciplinary training, mentorship, and practical experiences (e.g., liver clinic visits), to foster a deep understanding of HCV and related health determinants and build research skills. Through international and domestic collaborations (e.g., with Germany's TRR179 Network, Australia's Kirby Institute, and the International Network on Health and Hepatitis in Substance Users (INHSU)), trainees were exposed to diverse research environments and researchers, which improved their research skills, grant-writing skills and advanced their career. All trainees (n=3) interviewed as part of this case study reported that this program has successfully supported the transition into academic and research careers and became independent investigators within the network. One trainee reported that training opportunities through CanHepC allowed them to develop strong research skills, and to establish a network of community members and researchers, contributing to their success in CIHR's Project Grant program and in obtaining a tenure track position, which would not have otherwise occurred.
RI is advancing knowledge that contributes to prevent the acquisition and control the transmission of HIV/AIDS and STBBI.
The RI is advancing knowledge through peer review publications and through the support of infrastructure and progress in self-testing. Indeed, findings from the survey of researchers and trainees indicate that three quarters of researchers (76% out of 53) and the majority of trainees (88% out of 16) reported having published peer reviewed journal articles. Moreover, nearly two thirds of researchers (65% out of 49) reported that their RI-funded project resulted in data sources and/or solutions to better understand and treat HIV/AIDS and STBBI.
The findings from the survey are consistent with program documents that demonstrate that between 2018-19 and 2022-23, the RI-funded researchers produced a total of 727 peer reviewed publications. A bibliometric analysis was used to validate the extent to which these peer reviewed publications acknowledged CIHR and/or RI-funded initiatives. To this end, out of 727 total publications, 695 publications were indexed in the Dimensions bibliometric database, and of these, 564 (81%) acknowledged CIHR. Given that the majority of publications acknowledged CIHR, this finding validates the accuracy of the self-reported number of publications by RI-funded researchers. Peer-reviewed publications are a key means to inform decision-making that advances the response to HIV/AIDS and STBBI.
In addition to peer reviewed publications, the case studies highlighted multiple examples of advancing knowledge that contribute to the prevention and control of the transmission of HIV and STBBI. These include bringing new STBBI self-testing kits to market with the goal of reaching the undiagnosed. Specifically, REACH 3.0's research of the blood-based INSTI HIV Self-Test produced data to support the approval of the self-test kit by Health Canada, in November 2020. The CanHepC Network developed infrastructure to generate comprehensive data on the full spectrum of HCV care and outcomes, enabling provincial-level assessments, tracking of individual progression from infection and diagnosis to linkage to care, treatment, and cure. This type of in-depth, province-wide analysis had not previously existed in Canada, underscoring CanHepC's role in advancing the understanding of HCV care trajectories and outcomes at a provincial level.
RI Researchers are engaging community members and knowledge users in research; however, opportunities exist for CIHR to strengthen engagement with key organizations
Representatives across different groups, including priority populations, people with lived experience, knowledge users, and other community members or organizations are meaningfully engaged in all phases of the research process. Surveyed researchers reported that their project involved knowledge users (80% out of 56), and community organizations (75% out of 56). When compared to unfunded applicants, who indicated that their proposed project proceeded in the absence of RI funding (n=15), a smaller proportion of these researchers reported the involvement of knowledge users (47% out of 15) and community members (40% out of 15). Moreover, surveyed researchers reported that knowledge users were most frequently involved in the dissemination of results (73% out of 45) and knowledge exchange (63% out of 45). Similarly, community organizations were most frequently reported to be involved in the dissemination of results (74% out of 42) and knowledge exchange (74% out of 42). This involvement helped to promote knowledge mobilization and translation within communities.
"I think focusing on having people living with HIV as part of the team and guiding the research is really important just for making sure that what we prioritize is in alignment with what patients prioritize."
The survey findings are supported by the key informant interviews. Indeed, all interviewed researchers (5 out of 5) and knowledge users (4 out of 4) reported that priority populations and people with lived experience were actively engaged in the RI-funded research. This involvement included designing or developing data collection tools, serving in advisory roles, and disseminating findings through means that are most relevant to the community (e.g., community presentations, infographics). All interviewed researchers (5 out of 5) and one knowledge user (out of 4) highlighted that community members were engaged in every stage of the research process and that this community involvement was key to the success of the project, as it helped to identify relevant needs and gaps for these populations.
A prominent example of meaningful engagement, particularly within communities disproportionately impacted by HIV/AIDS and STBBI is the Feast Centre's engagement with a Council of Elders. The council, which includes elders from across Canada, representing First Nations, Inuit, and Métis, guides the Feast Centre in every aspect of its work, including representation within their governance structure (CIHR, 2021d). Additionally, the Canadian HIV Cure Enterprise (CanCURE) provides another example of meaningful engagement resulting in the implementation of a novel project with the potential to change professional practices (see project highlight box below).
Community engagement in CanCURE 2.0 End of Life Project
CanCURE 2.0 End of Life research project is a major research initiative focused on studying HIV reservoirs in human organs donated by PWLE for end-of-life research purposes. This project has actively engaged community members, including the families and friends of patients, recognizing the importance of family perspectives. Notably, CanCURE conducted a study to gather the perspectives of family members or next of kin regarding the rapid autopsy study. With community support, CanCURE 2.0 developed culturally safe and ethical guidelines and consent forms to guide the implementation of the autopsy project.
"A lot of great things have been done by various organizations in the federal initiative. CIHR being an elite player in research, and people are disappointed that kind of familiarity [with CIHR] has gone away."
Although the RI-funded researchers meaningfully engage with community members from priority populations in all phases of the research process, there are opportunities for CIHR to engage with organizations to coordinate efforts that advance key research priorities, build capacity for priority populations and advance knowledge mobilization efforts. Overall, some respondents from CIHR management and partner organizations (n=4 out of 10) observed that CIHR was missing opportunities to engage and establish partnerships with community organizations. For example, two representatives from CIHR management and partners (n=2 out of 10) observed that CIHR was absent from the largest Canadian conference on HIV/AIDS research hosted by the Canadian Association for HIV Research (CAHR), which CIHR could have leveraged as a means to engage with researchers from key communities to better understand barriers and challenges faced by these researchers. Here, it is important to note that many key informants from CIHR management (n=4 out of 7) acknowledge that proper engagement with priority populations requires in-person approaches, which is challenging given limited operational resources available to the RI.
Additionally, some respondents from both partners and CIHR management (n=3 out of 10) highlighted the opportunity to better coordinate engagements with both governmental and non-governmental organizations like PHAC, SSHRC, CAHR and CATIE to better support knowledge mobilization events and co-funded initiatives aligned with the RI's Strategic Plan (2022-2027).
The RI is strengthening the capacity of priority populations to participate in research
The RI is strengthening the capacity of people with lived experience, members of communities disproportionately impacted by HIV/AIDS and STBBI, other community members and organizations for greater participation in research, particularly community-based research. Almost all surveyed researchers (84% out of 56) reported that they have provided training and capacity building support to community members or people with lived experience. A notable example, from the document review, is the RI supported the EPIC Project implemented by a community organization in Quebec. This project trained members of priority populations, such as people who use drugs and sex workers, to conduct interviews. This training and capacity building effort supported individuals with lived and living experience to engage as Peer Researchers and Peer Research Assistants in other RI-funded projects (e.g., REACH CBR projects). For one person with lived experience, interviewed as part of the case study, the training provided strengthened their ability to both participate in research as well as understand and use research findings as it relates to comorbidities and navigating healthcare services.
The Waniska's capacity building activities include the use of culturally grounded frameworks and methodologies in research, to strengthen research capacity of researchers and communities. Case studies highlighted that the Waniska Centre is promoting a Two-Eyed Seeing approach in research combining culturally grounded tools and frameworks with Western techniques (see project highlight box below). It emphasizes human-centered approaches that integrate culture and spirituality, recognizing broader concepts of medicine, including spiritual healing, seen as essential in preventing infections and supporting those living with HIV/AIDS or STBBI.
Waniska promoted indigenous specific and community-centered research
The Waniska Centre has been instrumental in enhancing the capacity of researchers and community members to conduct Indigenous specific and community-centered research using culturally grounded frameworks and methodologies in a Two-Eyed Seeing approach. This approach was applied in the Keeping our Fires Together project, which developed and delivered a tele-monitoring program from HIV and HCV peer support workers across Saskatchewan. The Two-Eyed Seeing approach of the project provides a community of care that offers peers ongoing support and wellness training, culturally responsive activities and case management.
RI is improving the availability and awareness of research findings
The RI is increasing the availability and awareness of knowledge to inform HIV/AIDS and STBBI responses through knowledge transfer and exchange products, including conference presentations, knowledge dissemination platforms and events (e.g., workshops, symposium, café scientifique), and social media.
A review of administrative data indicates that between 2018-19 and 2022-23, RI-funded researchers produced 4,452 conference presentations. This finding is consistent with survey data which confirms that most researchers (87% out of 53) and three quarters of trainees (75% out of 16) presented research findings at national conferences, with many researchers (68% out of 53) and trainees (68% out of 16) also presenting at international conferences. While the majority of key informants across respondent categories (n=15 out of 19) agree that the RI is disseminating knowledge through conference presentations, some researchers and knowledge users (n=5 out of 9) highlighted that RI-funded research has improved the awareness and availability of research findings within communities through podcasts, webinars/symposia, Café Scientifiques, infographics and social media.
Examples from the case studies further supported these findings. For instance, the AHA Centre has increased availability and awareness of Indigenous HIV and STBBI knowledge through website engagement, community workshops on Indigenous Ways of Knowing and Doing (IWKD), hosting the Journal of Indigenous HIV Research (JIHR). This journal publishes international peer reviewed CBR research focusing on Aboriginal HIV and AIDS, which to date has 12 published volumes. Additionally, AHA disseminated knowledge through community bulletin board, bi-monthly newsletters distribution, events such as the Wise Practices GatheringFootnote 4, and social media (e.g., 285 Friends on Facebook, 790 followers on X reported in 2021) (CIHR, 2021f). The case studies also found that the RI is supporting the awareness and knowledge of HIV/AIDS and STBBI risk factors and stigmatizing behaviours through initiatives that address health inequity, stigma, and social determinants of health. A notable example is the You Matter Collaboration (CTN, 2023)Footnote 5. This initiative was developed in response to the disproportionately high rates of STBBI among incarcerated people in Canada and the significant barriers they face in accessing care. The initiative focuses on identifying strategies to address these barriers to improve access to STBBI testing and linkage to care for incarcerated individuals. It engaged key priority populations and stakeholders, including incarcerated individuals to promote equitable access to non-stigmatizing STBBI testing and care within British Columbia's correctional system. The initiative has led to policy recommendations and guidelines for STBBI testing and linkage to care in BC's provincial correctional facilities.
RI research is informing policy and guidelines in Canada.
Evidence indicates that the RI-funded research has informed policy and guidelines in Canada and abroad. An altmetric analysis performed on RI publications from 2018-19 to 2022-23, found that out of 680 publications indexed in the AltMetric database, 28 RI peer-reviewed publications across all funding streams and the CanHepC were cited in a total of 35 documents. Of the 28 peer reviewed publications, 23 were cited in a policy document, 4 were cited in two policy documents, and 1 peer review paper was cited in four policy documents (Figure 5: Number of RI publications by citation in policy). A similar analysis was conducted for RI-funded publications and patents. The altmetric analysis found that a total 18 RI-funded publications were cited in 41 patents (Figure 6: Number of publications by citations in patents). Of the 41 citations, most of the publications (n=13 out of 18) were cited in one patent, while notably one publication from the biomedical and clinical stream was cited in 16 different patents.
The survey findings are consistent with the altmetric analysis findings. Just under one-third of researchers (30% out of 50) reported that they were aware of findings from their funded research projects being cited in policy documents; whereas just over one-third of researchers (38% out of 49) reported that their research findings had contributed to informing decision-making in other areas. Further, the views of a couple key informants (n=2 out of 8) from researcher and partners categories also noted the RI's contribution to informing policy.
The case studies highlighted examples of RI-funded research informing guidelines and policies. A notable example is the Canadian PreP and nPEP treatment guidelines published in the Canadian Medical Association Journal. These guidelines were developed, updated and disseminated by CTN researchers through collaboration with policy makers, healthcare providers, pharmaceutical companies, and community organizations (e.g., CATIE, CAAN, CMA, CANAC). Professional organizations, including the Canadian Medical Association (CMA) and the Canadian Association of Nurses in AIDS Care (CANAC) who participated in the development of the guidelines, have contributed to their dissemination within practitioners. These guidelines have significantly influenced government policies and supported the implementation of programs across multiple Canadian provinces, including Alberta, Ontario, British Columbia, Saskatchewan and Quebec. Further, a case study showed that CanHepC's research has also influenced national clinical guidelines and policies both at the federal and provincial levels (see project highlight box).
CanHepC's Blueprint has impacted policy and guidelines
CanHepC developed a Blueprint for Hepatitis C Elimination in Canada through an inclusive process, synthesizing CanHepC research to provide adaptable policy recommendations across four key areas: priority populations, prevention, testing and diagnosis, and care and treatment. The Blueprint is already informing Correctional Services Canada health programming guidelines and in 2023, the Ontario Ministry of Health adopted it to guide its response to hepatitis C elimination. Regional roadmaps have been created to contextualize the Blueprint with an Indigenous-focused roadmap addressing health in Manitoba, Saskatchewan, and Alberta.
RI research is informing clinical practices and service uptake in Canada.
Findings from multiple lines of evidence indicate that the RI-funded research projects have informed clinical practices and service uptake in Canada. Just over one-third of surveyed RI researchers (34% out of 47) reported that their funded projects contributed to improving the efficiency and efficacy of clinical solutions to address HIV/AIDS and STBBI. Interview findings across respondent categories were consistent with this survey finding, with some reporting that RI research has developed interventions and diagnostic measures (n=5 out of 19) and informed practitioners and care providers (n=4 out of 19).
Case studies further illustrated these impacts, highlighting RI-supported initiatives such as REACH's HIV self-testing initiatives, including the I'm READY program Dried Blood Spot testing, Point of Care Tests for Syphilis and HIV (PoSH), and Approach 2.0 Study. These initiatives improved access to HIV self-test kits, allowing participants to connect to care through trained peer navigation support or online resources. Over 200,000 self-testing kits were distributed through over 400 agencies across Canada, with a goal of increasing HIV status awareness and connecting people to care, ultimately aiming to reduce HIV transmission. For example, REACH's PoSH study has newly diagnosed six people with HIV (CIHR, 2021g), based on data available at the time of this evaluation (see project highlight box below).
Advancing HIV self-Testing in Canada
RI-supported REACH Centre led a Canadian field study of the blood-based INSTI HIV Self-Test from bioLytical Laboratories. This study provided evidence of the test diagnostic performance and usability, used to support the license application of the test to Health Canada, which was approved in November 2020. The INSTI HIV self-test is now implemented in the REACH's I'm Ready program, a major national HIV self-testing research program, which provides free HIV self-test kits through a mobile application and offers peer navigation support for participants. Data available at the time of this evaluation indicates that the I'm Ready has registered more than 1,300 participants nationwide, spanning every province and territory. The program has equipped 17 Peer Navigators to provide support to participants throughout the testing process. 33% of individuals under 30 in the I'm Ready program were first-time testers, indicating increased access to testing.
There is limited evidence of RI research preventing new infections although RI research activities are making contributions toward prevention and reduced transmission.
The RI is contributing to the prevention of new infections and reduced transmission through increased access to self-testing. This supports the Government of Canada's Five-Year Action Plan on STBBI, which emphasizes early detection of STBBI through testing to enable timely diagnosis and treatment, improved health outcomes, prevention of long-term harms and reduced spread of STBBI (PHAC, 2024b). However, additional time and data are required to fully assess the extent of the RI's contribution.
Some researchers reported via the survey (29% out of 49, M = 2.84, SD = 1.2524) that their funded research had directly led to prevention or reduced transmission of new HIV and STBBI infections to a great or very great extent. This was supported by an interviewed researcher who highlighted the RI's important role in promoting culturally safe testing practices that increased access to testing and testing rates in Canada, consistent with findings from case studies on self-testing, presented above. However, several key informants (n=4 out of 9) across researcher and knowledge user categories noted limited evidence of long-term impact of RI-funded research focused on prevention and transmission.
There is evidence that RI research activities are contributing to improving the quality of life for PWLE, however, it is too early to assess this impact.
The RI research activities are contributing to improved quality of life for people living with HIV and/or STBBI. Approximately half (49% out of 49) of surveyed researchers reported that their HIV/AIDS and STBBI funded research had directly led to improved quality of life for people with HIV and/or STBBI to a great or very great extent. This was confirmed by several key informants across researcher, knowledge user and partner categories who emphasized that the RI has positively impacted the quality of life for people with lived experience by raising awareness (n=4 out of 12), improving service delivery (n=2 out of 12), addressing stigma and discrimination (n=3 out of 12), and creating support networks among study participants (n=1 out of 12).
These findings are consistent with findings from case studies. A notable example is the REACH study focused on the social determinants of health: the Positive Living, Positive Homes study. This study was conceived from the community's identification of housing as a critical health determinant for people living with, or at risk of HIV and AIDS. The study resulted in the development of an HIV Housing Toolkit which provides information about accessing and maintaining housing in BC for people living with HIV, while supporting service providers in helping their clients access and maintain housing. However, it is too early to assess the full impact of RI-funded research on improving the quality of life for PWLE.
CanHepC research is also contributing to slowing disease progression and improving the quality of life for PWLE. CanHepC is focused on reducing disease progression in high-risk populations by identifying undiagnosed individuals with HCV. Their research has generated prevalence estimates of undiagnosed individuals with HCV across various priority populations for Quebec, Ontario, and British Columbia. CanHepC is also evaluating the impact of birth cohort screening in BC on diagnosis rates and elimination targets. Additionally, the initiative addresses barriers to care and investigates the long-term health implications of HCV infection and treatment outcomes, using existing cohorts to analyze liver and non-liver outcomes to improve the quality of life for PWLE.
RI is contributing to the global efforts to reduce the spread of HIV/AIDS and STBBI, however, additional time and data are required to assess this impact.
The RI is contributing to the global efforts to reduce the spread of HIV and STBBI, however the extent of these contributions is unclear. Additional time as well as data linking of RI-funded research outcomes to global public health data would be required to better assess the extent of the RI's contributions.
The RI is informing global policies and fostering international collaboration. The altmetric analysis found 20 citations of RI-funded research in 18 international policy documents. This aligns with document review highlighting the examples of CTN and REACH contributing to WHO policy documents (e.g., REACH work on HIV self-testing informed WHO policy on HIVST). Additionally, case studies noted that the AHA centre has fostered international collaboration by securing funding for a multi-country project with Indigenous researchers, enhancing Indigenous health research capacity and creating a platform for global Indigenous advocacy through the International Indigenous Working Group on HIV/AIDS (IIWGHA). This effort demonstrates commitment to facilitating knowledge exchange and collaboration on a global scale, thereby extending the reach and impact of Indigenous knowledge uptake and application in health policy and practice.
CanHepC is also playing a crucial role in the global effort to combat HIV and STBBI through its collaboration with the NIH on HCV vaccine development. CanHepC also shares its expertise globally, including presentations at the US Centers for Disease Control and Prevention (CDC) on STBBI care models, and has formed partnerships with institutions like Germany's TRR179 Network and Australia's Kirby Institute, enhancing its global visibility. This has led to successful early career researchers and produced leaders in the field, such as the president of INHSU, further strengthening international collaborations with INHSU.
The COVID-19 pandemic negatively impacted the RI delivery and performance; however, RI management worked to mitigate the impacts by providing support to researchers.
Findings indicate that the COVID-19 pandemic had a negative impact on the design, delivery and performance of the RI, but the impact was mitigated by the RI's ability to provide operational supports to researchers. Document review highlighted that the COVID-19 pandemic caused delays in the development of key program documents, including the RI Strategic Plan 2022-2027. Additionally, strategic funding opportunities were temporarily paused and the RI prioritized COVID-19 and HIV/AIDS and STBBI intersectional research. The pandemic also disrupted in-person events such as conferences, knowledge dissemination activities, ceremonies for Indigenous peoples, annual meetings and workshops, limiting opportunities to networking and collaboration.
These findings were supported by survey and key informant interviews. Indeed, funded researchers reported via survey that the COVID-19 pandemic impacted their conference presentations (72% out of 49, M = 3.92, SD = 1.17), production of outputs and publications (53% out of 49, M = 3.59, SD = 1.27), access to research resources (53% out of 49, M = 3.49, SD = 1.43), and collaborations (49% out of 49, M = 3.5, SD = 1.27) to a great or very great extent. Moreover, just over one-half of key informants across groups (n=11 out of 19) noted that research had been negatively impacted due to delays in funding and other research processes, challenges in recruitment and engagement with the community.
However, more than one-half of key informants (n = 10 out of 19) noted that the research community had been resilient, mitigating some of the challenges by pivoting to online platforms (n = 8 out of 19), or focusing on COVID-19 related research to access funding opportunities (n = 3 out of 19). Additionally, document review highlighted that the RI provided operating financial support and time accommodation to mitigate the effect of the pandemic. This was confirmed by some members of CIHR Management (n = 3 out of 7) who noted that the RI provided extensions to researchers that had a positive impact on continuation of research.
Conclusions and Recommendations
Conclusions
Relevance
The evaluation found that the RI addresses an ongoing need to support HIV/AIDS and STBBI research. However, an expanded focus on other STBBI, coupled with the persistent health challenges posed by HIV/AIDS and STBBI, particularly among key populations that are disproportionately impacted, as well as emerging research needs (e.g., comorbidities and HIV/AIDS and STBBI) all underscore the continued need to support HIV/AIDS and STBBI research.
The RI is aligned with the roles and responsibilities of the federal government, as outlined in the Pan-Canadian STBBI Framework for Action, to facilitate research and innovation to understand STBBI transmission and to support the development of new diagnostic tools. The RI is directly aligned with the role of CIHR, as outlined in its Act, to support a multidisciplinary research agenda, address emerging health threats and promote knowledge dissemination to improve the health of Canadians.
The RI aligns with the priorities of both the federal government and CIHR. CIHR is the lead department for research related actions in the Government of Canada's Five-Year Action Plan on STBBI; thereby, aligned with federal government priorities. Additionally, the RI is aligned with the priorities of CIHR's current Strategic Plan (2021-2031). Finally, the RI's activities and investments are guided by its Strategic Plan (2022-2027), which informed the renewal of the Government of Canada's Five-Year Action Plan 2024-2030, thereby ensuring continued alignment with federal government priorities.
Design and Delivery
The RI's funding streams facilitate the achievement of its intended objectives by allowing the initiative to invest in multidisciplinary HIV/AIDS and STBBI research and adapt to evolving research needs. Challenges have been identified in the CBR funding stream as it relates to the development of research capacity within priority populations that are disproportionately impacted by HIV/AIDS and STBBI. Here, CIHR should make changes to its funding mechanisms to remove funding barriers by considering expanding eligibility requirements for applicants and community organizations, increasing the use of funding specific research streams, and/or adopting funding equalization approaches.
The RI has implemented an effective governance structure through CHASRAC. There is clear evidence that CHASRAC, through the use of its sub-committees for CTRN and CBR, is able to provide timely advice to the Scientific Director of III and CIHR's Vice-President Research Programs. This has enabled the RI to adapt to changes in the research environment such as evolving needs in the clinical trial, and identify risks to the RI, such as low application pressure in the Indigenous Stream for CBR and propose mitigation strategies.
Available data indicates that the RI is being delivered efficiently, with the estimated operating expenditures less than allocated expenditures. The financial data review found that CIHR spent on average, 59% of the RI's operating budget allocated in the program authorities, which has been associated with observed challenges in the implementation of key activities such as partnership and community engagement. Improvements to the monitoring of operational expenditures are needed as CIHR lacks a robust method for tracking FTEs associated with RI activities. Finally, there is limited evidence that progress and annual reports are contributing to informing program decision-making. Rather, there is evidence that reports are used to track investments, however gaps exist in the available reporting mechanisms that do not allow for annual monitoring and the communication of impacts.
Performance
The RI is funding research in priority areas across all four of its funding streams, and since 2018-19 has been supporting research on other STBBI by expanding the scope of existing initiatives (e.g., CTRN) and through targeted funding opportunities. The RI is building capacity to conduct research in HIV/AIDS and STBBI by supporting trainees with professional development and networking opportunities that contribute to successful grant applications and career advancement, however key challenges in supporting early-career researchers remain.
The RI is advancing knowledge that contributes to the prevention and control of new infections, increasing the awareness of research findings, and contributing to building capacity for priority populations. Researchers are engaging community members (including people with lived experience) in all phases of the research process, however there are opportunities for the RI to strengthen engagement with organizations in order to better understand the needs and barriers faced by communities disproportionately impacted by HIV/AIDS and STBBI. These engagements often necessitate in-person approaches, which is a challenge for the RI given the limited operational expenditures. Additionally, strengthening partnerships with governmental and non-governmental organizations such as PHAC, SSHRC and CATIE would allow to better coordinate knowledge mobilization efforts and support co-funded initiatives aligned with the RI's strategic plan.
The evaluation provides evidence that RI-funded research is informing policy and guidelines, impacting clinical practices and increasing service uptake. Notable examples include the advancement of self-testing, particularly among priority populations, informing guidelines for PrEP and NPEP treatments as well as elimination strategies for hepatitis C at the provincial level.
Finally, the RI research is contributing to the prevention and transmission of HIV/AIDS and STBBI, enhancing the quality of life for those impacted, as well as contributing to global efforts to reduce the spread of HIV/AIDS and STBBI. Although progress is being made toward achieving these long-term outcomes, more time is needed to fully achieve and assess the impact of the funded research.
Recommendations
The evaluation makes four recommendations aimed at improving the performance of the HIV/AIDS and STBBI Research Initiative to achieve its expected results.
- HIV/AIDS and STBBI to address ongoing and emerging research needs.
- CIHR should update the RI funding mechanisms to minimize application barriers and enhance the capacity of early career researchers and populations disproportionately impacted by HIV/AIDS and STBBI to access RI funding.
- CIHR should strengthen engagement with government and non-governmental organizations to:
- Advance research and build capacity for priority populations disproportionately impacted by HIV/AIDS and STBBI.
- Coordinate knowledge mobilization of RI-funded research to maximize its use by healthcare and community organizations.
- CIHR should improve the availability and monitoring of:
- Operating and maintenance expenditure data, specifically direct salary costs.
- Performance reporting of RI-funded research to better inform decision-making and communicate the impact.
Appendix A: Tables
Table 1: CIHR Allocated (based on TB submissions) and Actual Operating Costs on RI, 2018-19 to 2022-23
Data for the 2022-23 Evaluation
| Planned Spending | 2018-19 | 2019-20 | 2020-21 | 2021-22 | 2022-23 |
|---|---|---|---|---|---|
| Total Annual G&A | 20,995,000 | 20,995,000 | 20,995,000 | 20,995,000 | 20,995,000 |
| Total Annual Operating | 1,501,220 | 1,501,220 | 1,501,220 | 1,501,220 | 1,501,220 |
| PWGSC Accommodation Costs (13%) | 78,780 | 78,780 | 78,780 | 78,780 | 78,780 |
| Total Planned Expenditures | 22,575,000 | 22,575,000 | 22,575,000 | 22,575,000 | 22,575,000 |
| Total FTEs | 10 | 10 | 10 | 10 | 10 |
| Planned Operational Efficiency | 6.65% | 6.65% | 6.65% | 6.65% | 6.65% |
| Operating and Maintenance Costs | 2018-19 | 2019-20 | 2020-21 | 2021-22 | 2022-23 |
|---|---|---|---|---|---|
| Reported FTEs | 5.7 | 5.9 | 6.3 | 5.9 | 5.8 |
| Direct Salary | 530,616 | 554,134 | 589,996 | 569,785 | 589,815 |
| EBP - 20% (27% starting in FY2019-20) | 106,123 | 149,616 | 159,299 | 153,842 | 159,250 |
| Accommodation - 13% | 68,980 | 72,037 | 76,700 | 74,072 | 76,676 |
| Non-Salary | 152,493 | 158,657 | 13,688 | 121,436 | 49,704 |
| Total Annual Operating Costs (Salary + non-salary) | 858,213 | 934,444 | 839,683 | 919,134 | 875,444 |
| Total G&A Expenditures | 20,012,443 | 20,975,728 | 19,799,645 | 21,916,427 | 20,332,297 |
| Total Expenditures | 20,870,656 | 21,910,172 | 20,639,328 | 22,835,561 | 21,207,741 |
| Actual Operational Efficiency | 4.11% | 4.26% | 4.07% | 4.03% | 4.13% |
Source: Treasury Board Submissions and CIHR Finance Data
Appendix B: Figures
Figure 1: CIHR Investment in HIV/AIDS and STBBI
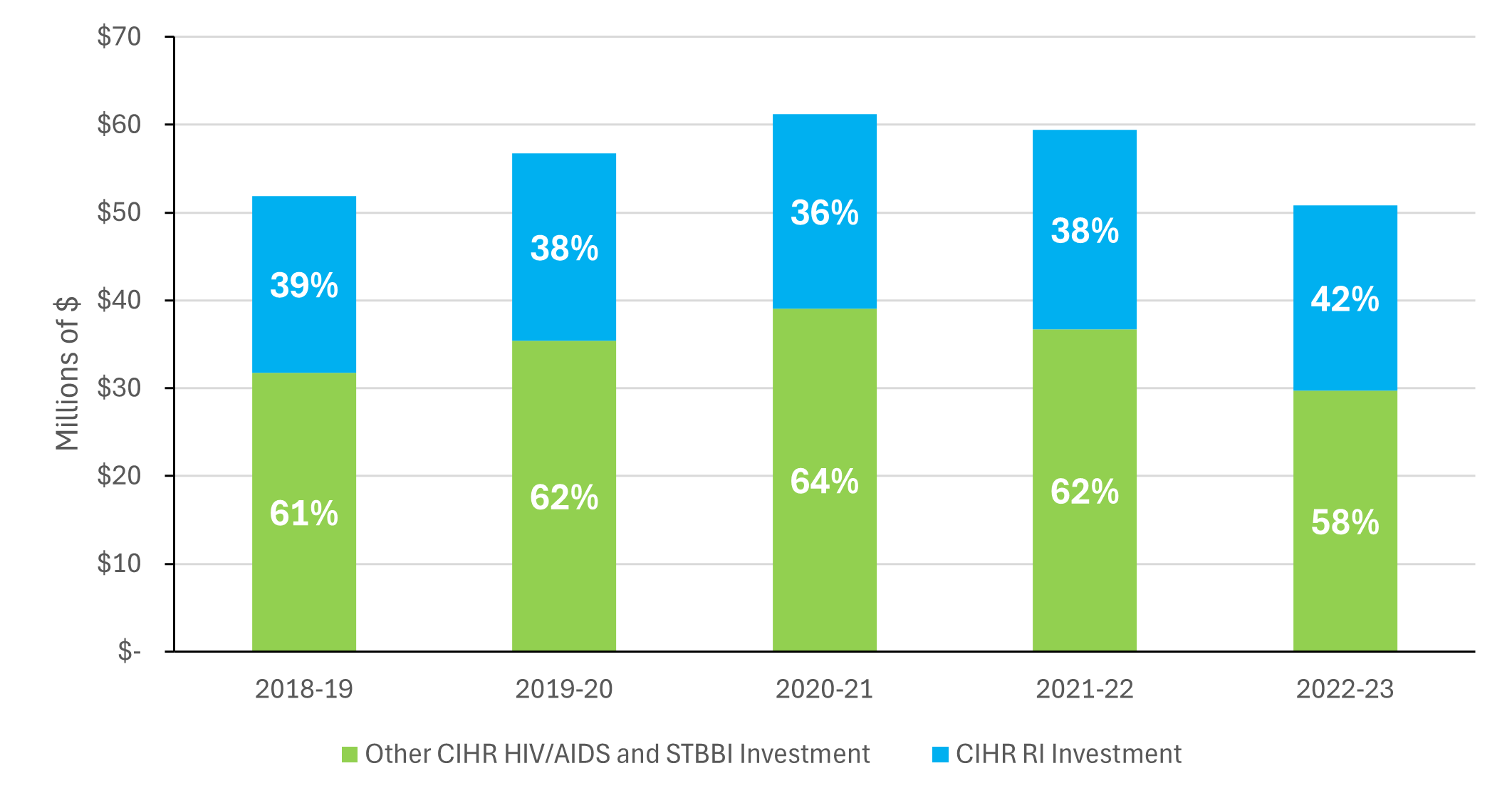
Figure 1 long description
| 2018-19 | 2019-20 | 2020-21 | 2021-22 | 2022-23 | Total | |
|---|---|---|---|---|---|---|
| Other CIHR HIV/AIDS and STBBI Investment | $31,776,201.57 | $35,411,840.43 | $39,060,892.00 | $36,731,499.00 | $29,731,745.00 | $172,712,178.00 |
| CIHR RI Investment | $20,141,442.00 | $21,309,161.00 | $22,128,655.00 | $22,661,205.00 | $21,100,809.00 | $107,341,272.00 |
Source: CIHR Electronic Information System (EIS) data, 2018-19 to 2022-23
Figure 2: CIHR RI Investment by Funding Stream
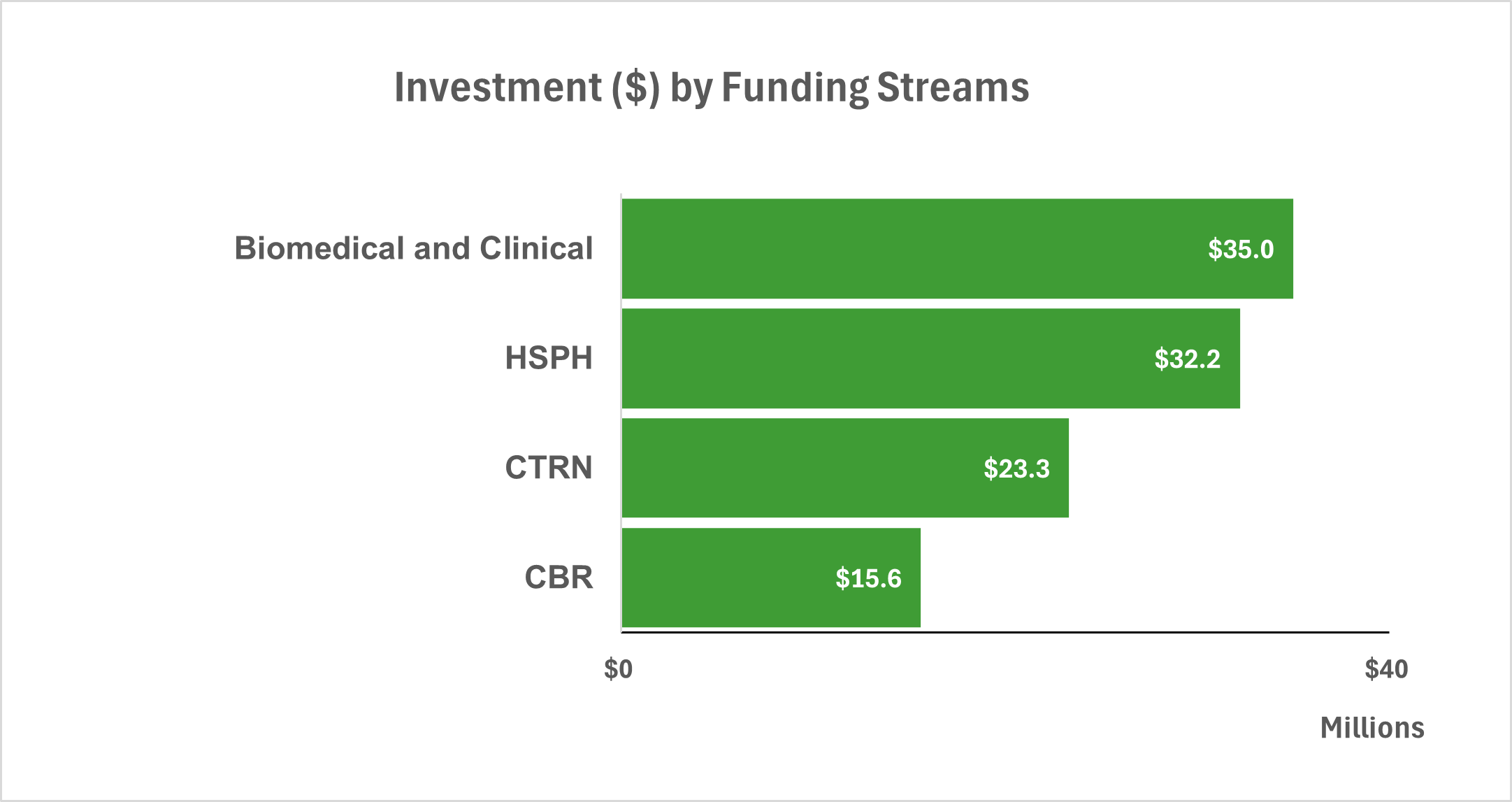
Figure 2 long description
| Investment by funding stream | |
|---|---|
| CBR | $15,607,449.00 |
| CTRN | $23,326,651.00 |
| HSPH | $32,217,317.00 |
| Biomedical and Clinical | $35,011,751.00 |
Source: CIHR Electronic Information System (EIS) data, 2018-19 to 2022-23
Figure 3: Top 10 countries on HIV/AIDS and STBBI research Specialization Index (2004-2022)
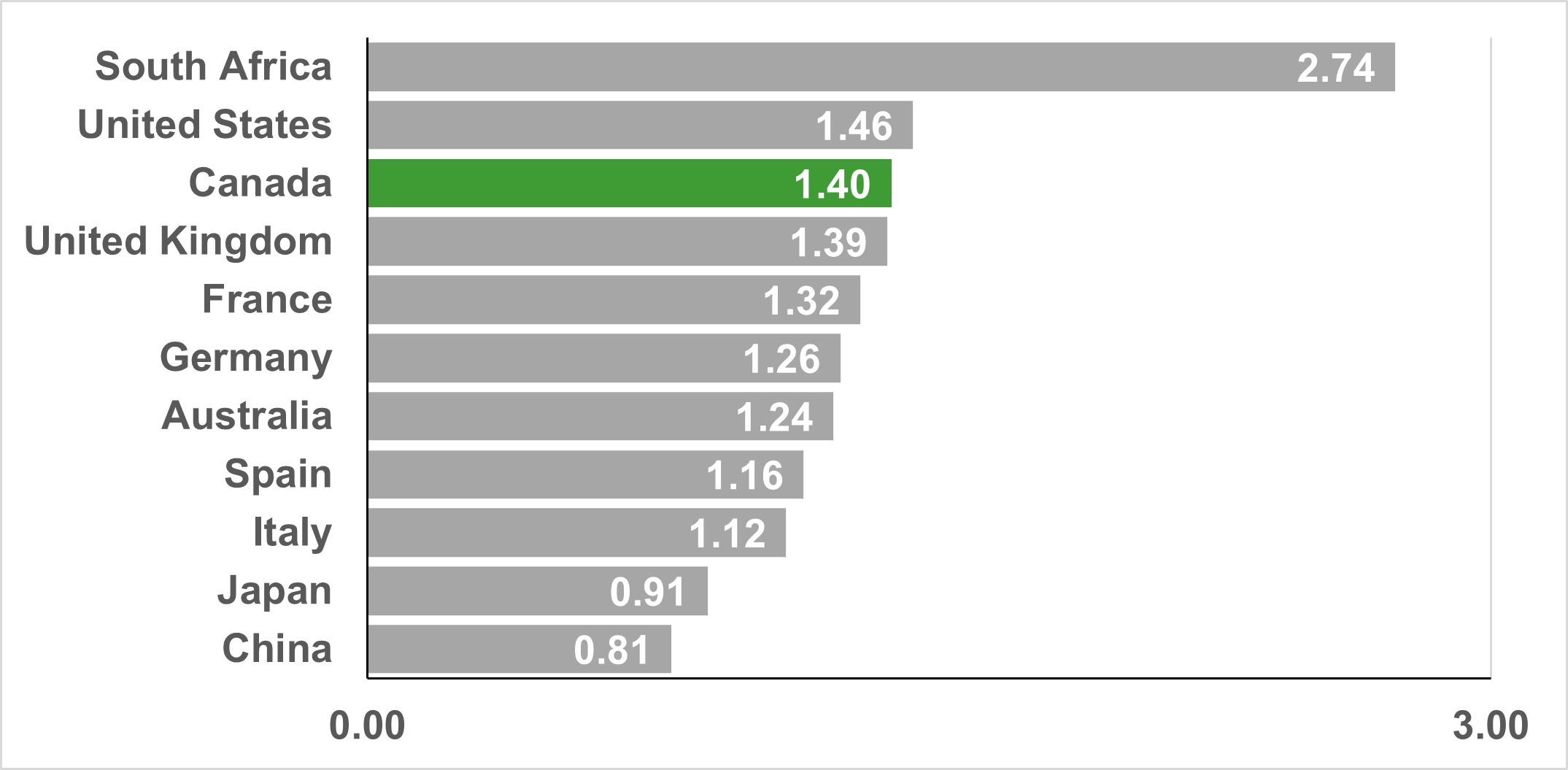
Figure 3 long description
| Country | Specialization Index |
|---|---|
| China | 0.81 |
| Japan | 0.91 |
| Italy | 1.12 |
| Spain | 1.16 |
| Australia | 1.24 |
| Germany | 1.26 |
| France | 1.32 |
| United Kingdom | 1.39 |
| Canada | 1.40 |
| United States | 1.46 |
| South Africa | 2.74 |
Source: Observatoire des sciences et des technologies (Clarivate Analytics – Web of Science).
Figure 4: Top 10 countries by the Average of Relative Citations (ARC) of Publications in All STBBI areas (2004-2022)
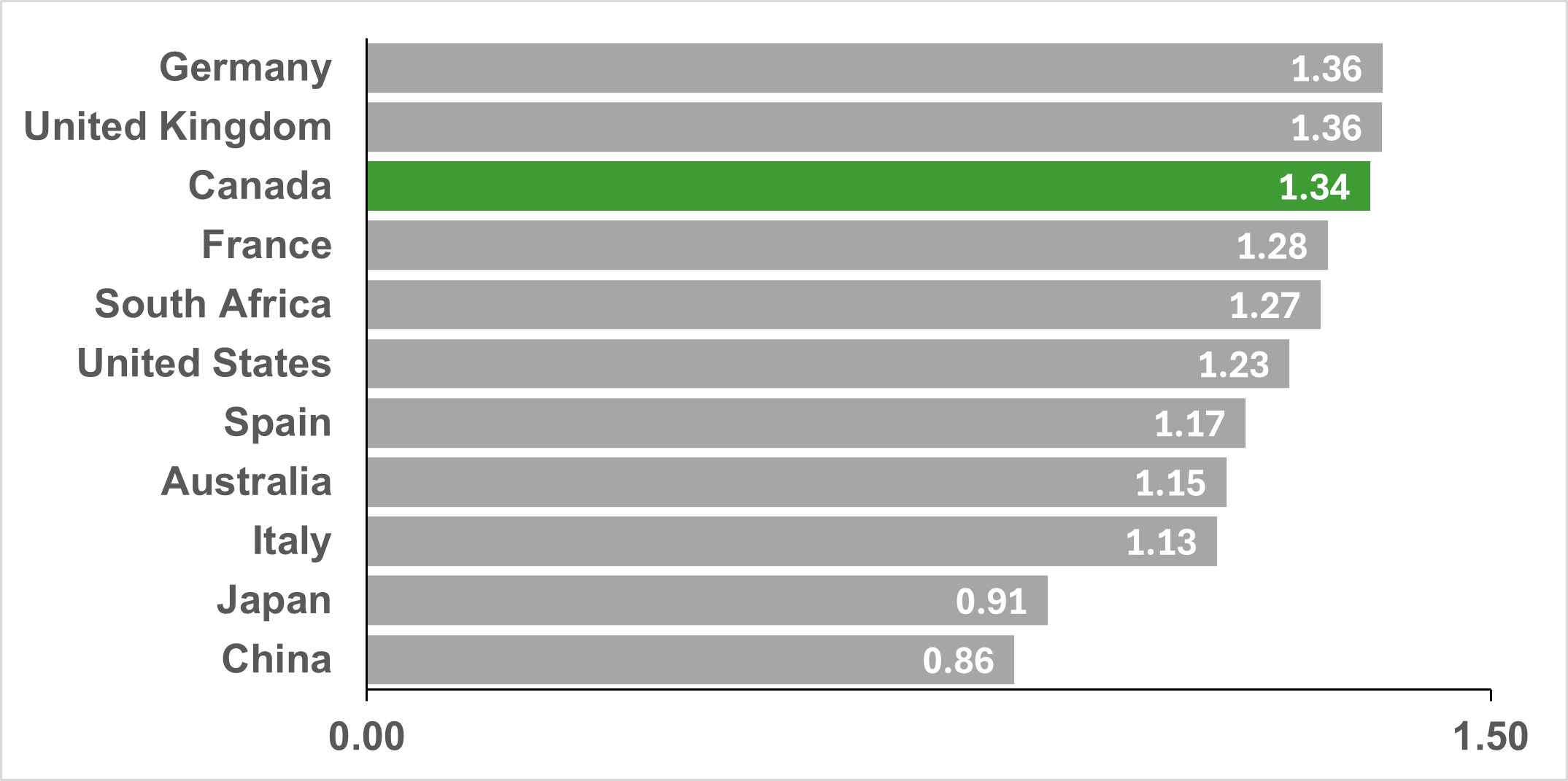
Figure 4 long description
| Country | Average of Relative Citations (ARC) |
|---|---|
| China | 0.86 |
| Japan | 0.91 |
| Italy | 1.13 |
| Australia | 1.15 |
| Spain | 1.17 |
| United States | 1.23 |
| South Africa | 1.27 |
| France | 1.28 |
| Canada | 1.34 |
| United Kingdom | 1.36 |
| Germany | 1.36 |
Source: Observatoire des sciences et des technologies (Clarivate Analytics – Web of Science).
Figure 5: Number of RI publications by citation in policy

Figure 5 long description
| Number of policy citations | Number of publications |
|---|---|
| 4 Citations | 1 |
| 2 Citations | 4 |
| 1 Citation | 23 |
Source: Digital Science – Altmetric Database
Figure 6: Number of publications by citations in patents
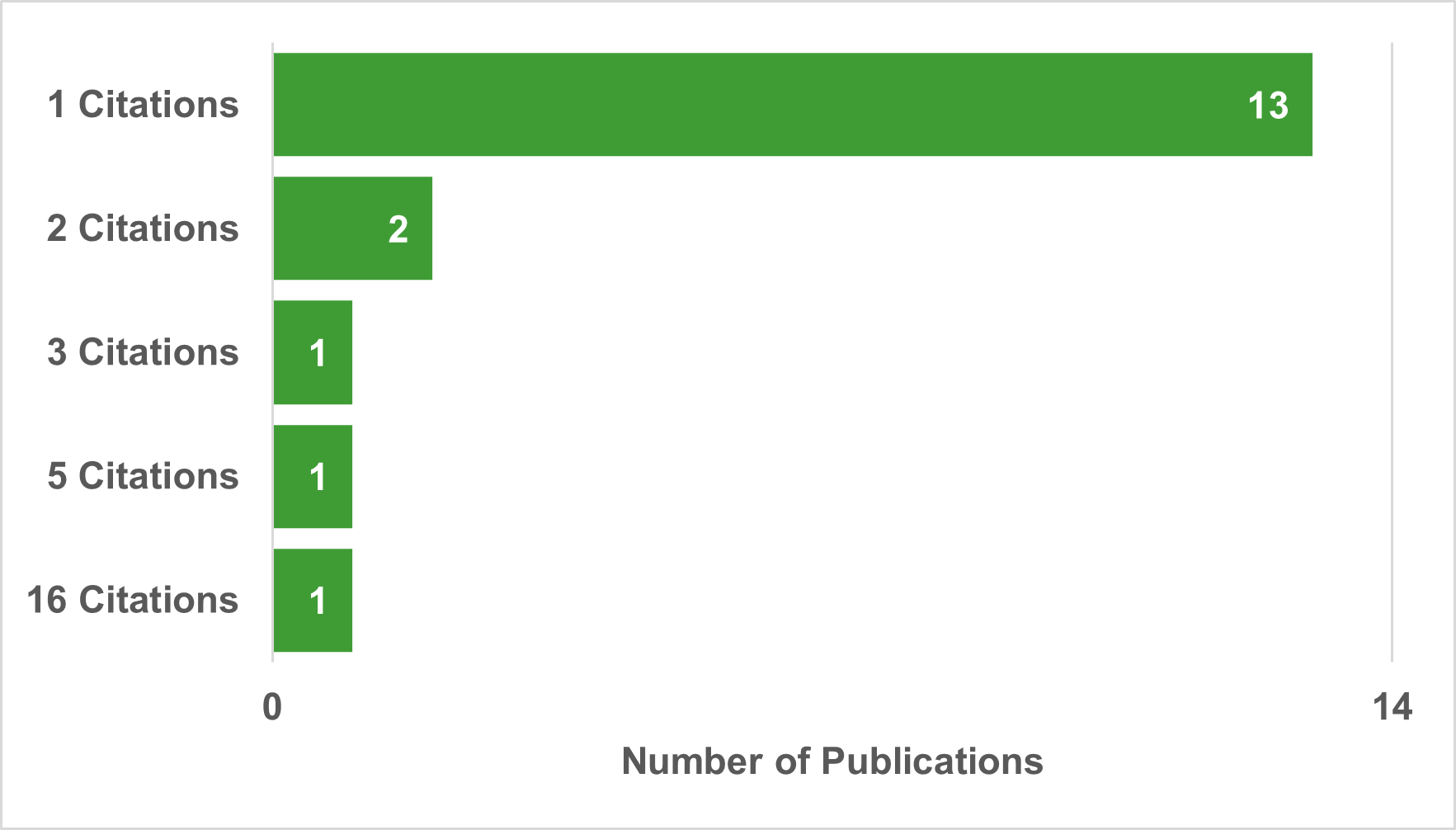
Figure 6 long description
| Number of patents | Number of publications |
|---|---|
| 16 Citations | 1 |
| 5 Citations | 1 |
| 3 Citations | 1 |
| 2 Citations | 2 |
| 1 Citations | 13 |
Source: Digital Science – Altmetric Database
Appendix C: HIV/AIDS & STBBI Research Impact Summaries
Impact Summary #1 – Canadian Network on Hepatitis C (CanHepC)
Research Overview
In 2015, CIHR-III and PHAC funded a National Hepatitis C Collaborative Network (Canadian Network on Hepatitis C [CanHepC]), with an investment of $4.5 million ($900k annually) during this evaluation period (2018-19 to 2022-23), to support research in two overarching streams: biomedical and clinical research; and research with direct public health relevance. Its overarching goal is to improve health outcomes for people living with hepatitis C at all stages of the cascade of care, closing the gap between knowledge and practices while training and building capacity in research.
Outcomes and Impacts
CanHepC has advanced knowledge through peer-reviewed publications, conference presentations, and other knowledge translation (KT) events via its annual Canadian Symposium on Hepatitis C Virus, which brings together Canadian and international researchers, healthcare practitioners, trainees, policymakers, community groups, and people with lived experience. CanHepC is building capacity through its training and mentorship program that has supported over 131 trainees, some of whom have become leaders in the field. Many have come full circle as independent investigators and co-applicants within the network. This success stems from the program's multidisciplinary training approach, practical experiences, and global collaboration opportunities.
CanHepC is informing policy and guidelines both nationally and internationally, with its Blueprint for Hepatitis C Elimination in Canada influencing health guidelines for Correctional Service Canada and Ontario's response to HCV elimination. The network's research has also contributed internationally to WHO, American and European guidelines. The network is making significant strides in improving hepatitis C outcomes in Canada through data linkage systems and mathematical modeling to estimate undiagnosed HCV cases in priority populations, addressing asymptomatic infection, and monitoring disease progression with provincial data to identify care gaps and reduce undiagnosed cases.
Impact Summary #2 – Canadian HIV Trials Network (CTN)
Research Overview
The Canadian HIV Trials Network (CTN) is a national network that focuses on accelerating the translation and uptake of new knowledge into clinical practice and guidelines for HIV/AIDS and other STBBI; as well as strengthening the capacity of researchers, building infrastructure, and delivering specialized expertise and services to facilitate clinical research, including trials. Established in 1990, the CTN has received $23.3 million in funding during the evaluation period (2018-19 to 2022-23) from the HIV/AIDS and STBBI Research Initiative (RI). It supports research teams in regions across Canada to include researchers, community members, and Indigenous representation.
Outcomes and Impacts
CTN has advanced knowledge through high-impact journal publications, more than 1700 national and international presentations, and substantive online and social media engagement. CTN has engaged in different activities with partner organizations, including conferences, workshops, and funding initiatives. CTN is building capacity by funding 110 postdoctoral fellows and supporting a network of over 160 clinical investigators. It also hosts bi-annual knowledge sharing meetings with community members, apprentices, trainees, postdocs, academics, and other researchers.
CTN is informing decision-making through its research being cited in nine patents (2018-19 to 2022-23), guidelines, and clinical applications. Notably, its contributions to the PrEP and nPEP treatment guidelines have influenced government policies and supported program implementation across multiple Canadian provinces. CTN has played a pivotal role in the development of global health policies, including WHO guidelines on HIV antiretroviral therapy (ART) regimens, and the Canadian guidelines on HIV pregnancy planning. Several clinical trials supported by CTN are currently testing new medications and combinations of medications aimed at treating individuals living with HIV and/or other STBBI.
Impact Summary #3 – CanCURE
Research Overview
The Canadian HIV Cure Enterprise 2.0 (CanCURE 2.0) team has received $6.3 million in funding during the evaluation period (2018-19 to 2022-23), within the Biomedical and Clinical Research stream of the RI. It focuses on studying HIV persistence during antiretroviral therapy and developing strategies towards a sustainable HIV remission. This project is a collaboration of leading Canadian HIV researchers, as well as knowledge users who present the perspective of individuals living with HIV. CanCURE research has mostly been about fundamental science research, and its projects have aimed to better understand the mechanisms by which HIV persists in human tissues (known as HIV reservoir) and develop cure approaches.
Outcomes and Impacts
CanCURE has advanced knowledge through studies and clinical trials, publications, presentations, and communications during annual meetings and conferences at local, national, and international levels. Awareness and research findings have been communicated to community through a series of "Café Scientifique", website and social media engagements, as well as development of communication materials in lay languages.
CanCURE 2.0 has strengthened the capacity of researchers, trainees, and community members, and has collaborated with partner organizations. CanCURE research is informing decision making by being cited in 11 patents (2018-19 to 2022-23) and in international policy documents. There is ongoing work to develop therapeutic strategies that target the persistent HIV reservoir with the potential to change professional practices in the HIV field. CanCURE has also been developing guidelines for End-of-Life HIV research as well as for the monitoring of analytical treatment interruptions.
Several ongoing clinical studies are investigating drugs and therapies to improve quality of life of people with lived experience.
Impact Summary #4 - REACH
Research Overview
Since 2009, the CIHR Centre for REACH has been a Canadian leader in collaborative, interdisciplinary, and community-driven HIV/STBBI services and population health research. During the evaluation period (2018-19 to 2022-23) REACH received $11.3 million in RI funding to support a nation-wide team to engage in innovative research, participatory evaluation, community-based research (CBR), and program/implementation science research in HIV, HCV, and STBBI. The aim of the network is reaching the undiagnosed, implementing and scaling up new testing options, strengthening connections to care, improving access to options for prevention, and ending HIV stigma.
Outcomes and Impacts
REACH has advanced knowledge through advancement of tools and new research methods, and has participated in 51 events including trainings, conferences, or workshops; produced a number of publications; and has disseminated knowledge via blog and social media posts as well as other unique KTE products such as videos, podcasts, promotional material, toolkits, and webinars. REACH is building capacity through efforts like Universities Without Walls which has supported over 100 fellows and is establishing a series of Indigenous-focused capacity building workshops.
REACH is informing decision-making through partnerships with community organizations and has bridged connections with industry and decision-makers across Canada. REACH has contributed to national and regional health policy and guidelines, with research findings cited in the WHO policy on HIV Self-Testing and several international guidelines. The REACH Centre is contributing to broader health impacts by introducing HIV self-tests to the Canadian market, distributing over 200,000 self-test kits to over 400 agencies nationwide. REACH has implemented community-based testing approaches, including dried blood spot testing in Indigenous communities and among Gay, Bisexual, and other Men-Who-Have-Sex-with-Men (GBMSM), as well as point-of-care testing using peer community workers. These efforts are allowing to reach high-risk individuals and those who are previously untested, enhancing access to early diagnosis and care.
Impact Summary #5 – Aboriginal HIV and AIDS Community-Based Research Collaborative Centre (AHA Centre) 2.0
Research Overview
The Aboriginal HIV and AIDS Community-Based Research Collaborative Centre (AHA Centre) 2.0, housed within the Canadian Aboriginal AIDS Network (CAAN), is a $1.6 million funding initiative since 2017 for a five-year term under the Community-Based Research (CBR) funding stream of the RI. This Centre supports HIV and AIDS CBR conducted in First Nations, Inuit and Métis communities across Canada. It aims to achieve five objectives: 1. Indigenous Ways of Knowing and Doing; 2. Communications; 3. Knowledge Translation; 4. Partnerships and 5. Support/Mentorship.
Outcomes and Impacts
The AHA Centre has advanced knowledge by creating the Journal of Indigenous HIV Research, a unique journal that publishes international peer-reviewed CBR research focusing on Aboriginal HIV and AIDS. The Centre has also shared 32 posters and presentations at regional, national, and international levels; published 10 research articles; and contributed to a book chapter. To disseminate knowledge more broadly, the Centre utilizes newsletters and social media, as well as its flagship KT event, the Wise Practices Gathering. The AHA Centre is building capacity by helping organizations secure funding (e.g., a $4.8 million grant for the FEAST Centre), facilitating capacity building events (e.g., CBR Peer Workshop and Wise Practices VII), establishing international collaborations, and supporting trainees in the area of Indigenous HIV CBR.
The AHA Centre is informing decision-making by shaping guidelines, including a WHO guideline on care and treatment of persons diagnosed with chronic hepatitis C virus infection. Through funding and events such as the Wise Gathering, the Centre has created a platform for individuals from research, healthcare, government, and policy sectors to share knowledge and experiences, further contributing to the uptake and application of Indigenous knowledge in health-related practices and policies.
Impact Summary #6 – Waniska Centre
Research Overview
The Waniska Centre is a $2.3 million funding initiative since 2019 for a five-year term under the Health Services and Population Health Research funding stream of the RI. It is an Indigenous-led centre for research on HIV, Hepatitis C Virus (HCV) and other sexually transmitted and blood borne infections (STBBI) focused on Saskatchewan (SK) and Manitoba. The Centre's aim is to engage Indigenous communities and people in research that is focused on Indigenous knowledge and the land to develop, explore, and scale up promising and wise practices, using both an Indigenous and Western lens, or Two-eyed Seeing approach.
Outcomes and Impacts
The Waniska Centre has advanced knowledge through publications, conference presentations, and in-person KT events, as well as through newsletters and its website. The Centre is building connections with community members through initiatives such as weekly sharing circles for people living with HIV/AIDS and STBBI, food and winter clothing drives, with local Elders, and Sask Stories: a community-driven project aimed at creating a web-accessible database of published and unpublished HIV and HCV programs, projects, and initiatives in SK. The Centre has built capacity by promoting learning pathways and certification opportunities for individuals interested in meaningful, Indigenous-specific, community-centered research, through the mâmâwihitowin ("gathering of people") initiative. It has also leveraged resources from the University of Saskatchewan to increase community organization's capacity to do research. Through Waniska's support, community organizations have secured further funding from PHAC for the Keeping Our Fires Together and Sask Stories projects. Furthermore, through the Centre's support, Nine Circles secured two grants, resulting in the establishment of the Village Lab, a local research hub.
The Centre has begun making progress towards informing decision-making through a partnership to develop Primary, Public, and Traditional Health Systems Frameworks and policies for addressing HIV, HCV, and STBBI in First Nations populations in SK, and through its work leading the development of National Indigenous and Prairie Region Roadmaps which outline actionable steps to enhance prevention, testing and treatment services, and aim to eliminate HCV as a public health threat.
References
- CIHR. (2004). HIV treasury board submission. Unpublished manuscript.
- CIHR. (2015). Strategic plan 2014-15 – 2018-19: Health research roadmap II: Capturing innovation to produce better health and health care for Canadians [ PDF (1.0 MB) ].
- CIHR. (2019). Message from the Scientific Director, III: HIV/AIDS and STBBI research update.
- CIHR. (2021a). Strategic plan 2021-2031: A vision for a healthier future [ PDF (4.1 MB) ].
- CIHR. (2021b). CHASRAC meeting minutes December 2021. Unpublished manuscript. CIHR. (2021c). CHASRAC meeting minutes March 2021. Unpublished manuscript.
- CIHR. (2021d). CHASRAC terms of reference. Unpublished manuscript.
- CIHR. (2021e). Strategic plan 2021-2031: A vision for a healthier future [ PDF (4.1 MB) ]
- CIHR. (2021f). Annual report for CIHR collaborative centres of HIV/AIDS community-based research: AHA. Unpublished manuscript.
- CIHR. (2021g). Progress report for REACHing for impact. Unpublished manuscript. CIHR. (2022a). CHASRAC meeting minutes September 2022. Unpublished manuscript.
- CIHR. (2022b). CIHR HIV/AIDS and STBBI research initiative strategic plan: 2022-2027 [ PDF (1.94 MB) ].
- CIHR. (2023a). BN for the director general, Initiative Management and Institute Support. Unpublished manuscript.
- CIHR. (2023b). CHASRAC meeting minutes January 2023. Unpublished manuscript.
- CTN. (2023). Annual progress report 2022-23. Unpublished manuscript.
- Government of Canada. (2000). Canadian institutes of health research act [ PDF (273 KB) - external link ].
- PHAC. (2018). A pan-Canadian framework for action: Reducing the Health Impact of Sexually Transmitted and Blood-Borne Infections in Canada by 2030 [ PDF (6.2 MB) - external link ].
- PHAC. (2019). Accelerating our response: Government of Canada five-year action plan on sexually transmitted and blood-borne infections [ PDF (1.66 MB) - external link ].
- PHAC. (2024a). HIV in Canada: 2023 Surveillance Highlights [ PDF (316 KB) - external link ].
- PHAC. (2024b). Government of Canada's Sexually transmitted and blood-borne infections (STBBI) action plan 2024-2030 [ PDF (1.51 MB) - external link ].
- WHO. (2022). Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030 [ PDF (3.9 MB) - external link ].
- WHO. (2024). HIV statistics, globally and by WHO region, 2024 [ PDF (303 KB) - external link ].
- Date modified: